How cost-effective is it to treat multidrug-resistant tuberculosis?
Every year on the 24th of March, World TB Day raises awareness of the global threat posed by tuberculosis. Although much progress has already been made in combatting the disease, challenges still remain. In particular, the rise of drug-resistant tuberculosis has made efforts to control the disease very difficult, and it is necessary to assess the effectiveness of different treatment strategies.
About a third of the world’s population has tuberculosis (TB), a bacterial infection caused by Mycobacterium tuberculosis. TB spreads from one person to another in airborne droplets from the throat and lungs. While in the majority of people the infection is latent, meaning that they carry the bacteria but are not ill or infectious, a significant proportion develop disease. TB affects the lungs and causes symptoms such as cough (sometimes with sputum and blood), chest pains, weakness, weight loss, fever and night sweats [1]. Despite the fact that TB is both preventable and curable, it currently ranks as the second leading cause of death worldwide from a single infectious agent after HIV, with 8.6 million new cases and 1.3 million deaths reported in 2012 [2]. The main brunt is borne by the world’s poorest people: 95% of all deaths from TB occur in low- and middle-income countries. The probability of developing TB is higher in immunocompromised people, such as those infected with HIV, malnourished, diabetic, or those who use tobacco [1]. The interventions most often used today are preventative measures such as vaccination, and treatment with antibiotics, which can be very cost-effective. However, poor adherence to the treatment can result in bacteria developing resistance to the antibiotics, giving rise to multidrug-resistant TB (MDR-TB). This article will discuss the various options for treating MDR-TB, as well as their cost-effectiveness.
Reassuringly, TB incidence and mortality worldwide are dropping. Following the World Health Organisation’s (WHO) Global Plan To Stop TB with several targets to be achieved by 2015 [4], TB incidence is falling in most parts of the world, and mortality has reduced by 45% since 1990 [3]. This success results from the implementation of the DOTS/Stop TB Strategy, launched by the WHO in the 1990s. Key elements of the strategy include improving TB detection by bacteriology, standardised treatment and sustained drug supply, and recording and evaluating patient data. DOTS (Directly Observed Treatment, Short-Course) is a six-month chemotherapy course using first-line antibiotics, where the administration of drugs is directly supervised by a trained individual to ensure the patient adheres to and completes the treatment [5]. The cost-effectiveness of DOTS has been researched in a number of countries. The principle of cost-effectiveness is to assess the cost of an intervention (compared to another intervention or compared to doing nothing) against the benefit to the population. This is typically expressed in $ per DALY (Disability Adjusted Life-Year) averted [6]. For more information on DALYs, please refer to the latest Giving What We Can blog post on this topic [7]. The cost-effectiveness of DOTS ranges from US$5 to $50 per DALY averted, except for Eastern and Central Europe, where the cost is higher [8, 9].
Despite the recent success in treating drug-sensitive TB, there is a threat to the TB control programme from bacterial resistance to first-line antibiotics. MDR-TB, caused by bacteria resistant to at least two of the most effective anti-TB drugs (isoniazid and rifampicin), is now a serious public health problem in several countries. At the moment, only 3.6% of new TB cases are multidrug-resistant, compared to 20% among previously-treated patients. However, in some countries such as Azerbaijan, Belarus, and some regions in the Russian Federation, the percentage is much higher (see figure). MDR-TB arises from poor compliance with treatment or incorrect use of drugs [3, 10]. Combatting MDR-TB is included in the Global Plan To Stop TB, which recommends testing for drug resistance in high-risk patients and treatment with second-line antibiotics such as amikacin and kanamycin. However, so far only 34 out of 107 countries have achieved the 2015 target of successfully treating ≥75% of MDR-TB patients. This may be in part due to low detection rates: only 18% of the estimated global MDR-TB cases were reported to the WHO in 2012 [10].
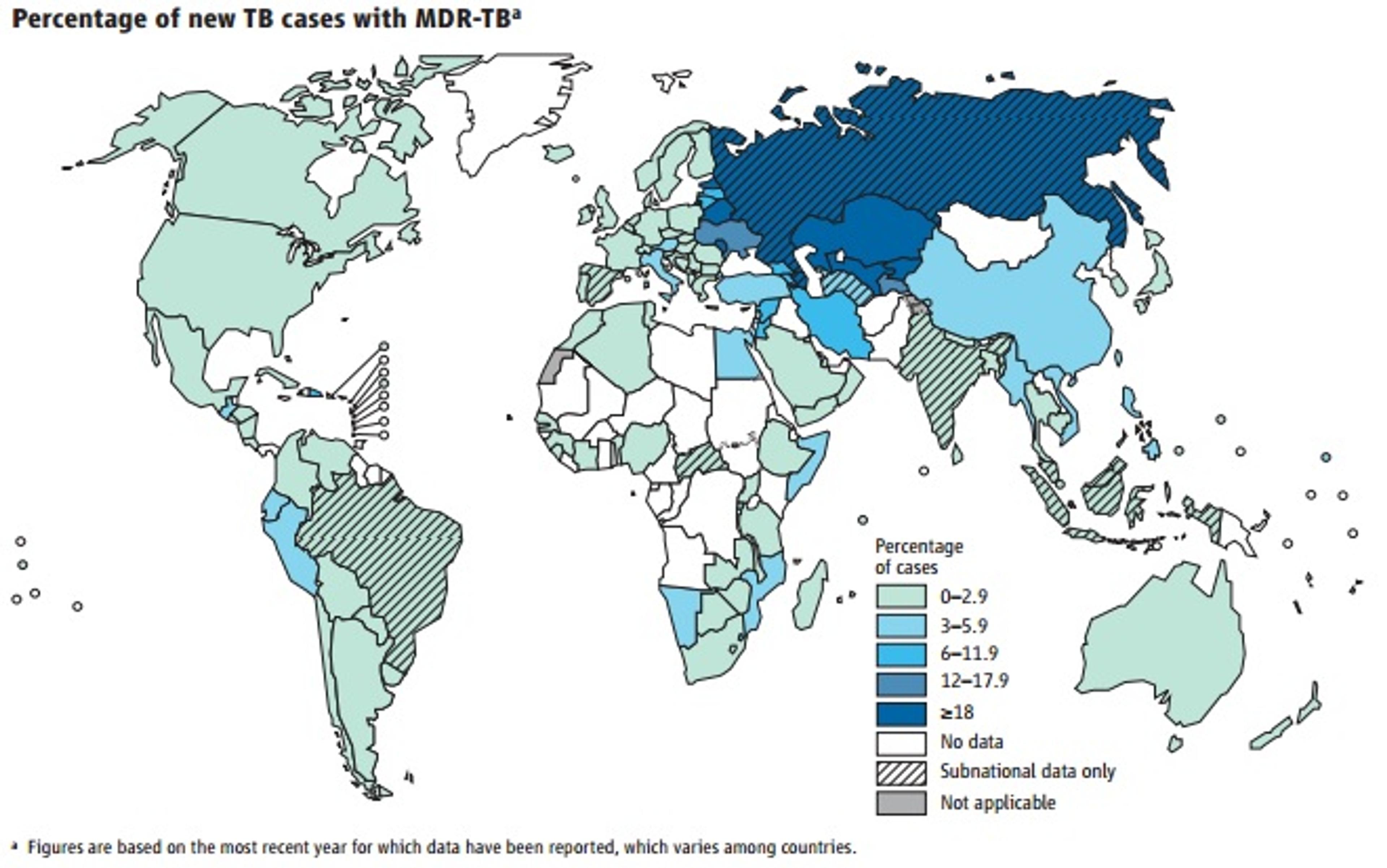
Figure: Percentage of new TB cases with MDR-TB, from the WHO Global TB Report 2013
Although second-line therapy is more effective at curing MDR-TB patients than DOTS, it is more expensive, requires longer treatment, and can have more side-effects [11]. It is therefore important to identify the most cost-effective models of treatment, especially in poorer regions where the burden is highest. The cost-effectiveness of different MDR-TB interventions has already been analysed for several low- and middle-income countries. One study evaluated five different treatment programmes used in Peru. These were DOTS, standardised second-line drug courses for previously-treated patients, with or without testing for drug resistance (STR1 and STR2, respectively), and individualised treatment following drug susceptibility tests for previously-treated cases or all TB cases (ITR1 and ITR2, respectively) [12]. With such studies, a range of assumptions must be made about the prevalence of MDR-TB in the country, the rate of transmission, and cure rate probability for each intervention. The results are gives as incremental cost-effectiveness, i.e. the net extra cost per net benefit of one strategy compared to another. Overall, DOTS-Plus strategies (testing a previously-treated patient for MDR-TB and treating them with second-line drugs) were more cost-effective than DOTS on its own. STR2 would prevent 4.8 deaths per 100,000 over 30 years, at an incremental cost of $990 per DALY averted compared to DOTS. STR1 provides the same benefit as SRT2 but at a higher cost, and is therefore less effective. The WHO considers an intervention to be cost-effective if the cost per DALY averted is lower than three times the per capita GDP of that country, and highly cost-effective if it is lower than one per capita GDP [13]. All things considered, ITR1 was determined to be the optimal strategy for Peru, preventing 0.9 deaths per 100,000 over a 30-year period at an incremental cost of $990/DALY averted compared to STR2 (below the $2,360 per capita GDP in Peru), or $6400/DALY averted compared to DOTS.
A more recent extensive review compared MDR-TB interventions in four regions: Estonia, Peru, Philippines and Tomsk Oblast in the Russian Federation [14]. While in Estonia and Tomsk patients were treated predominantly in hospital, Peru and the Philippines employed mostly ambulatory care. In terms of treatment strategies, Peru used a standardised regimen, while the Philippines, Estonia and Tomsk all used individualised regimens with drug resistance tests. Due to the higher costs of hospital stays compared to ambulatory care and differences in drug costs, the cost of treatment per patient varied from $2423 in Peru to $14,657 in Tomsk. The costs per DALY averted were $163 in Peru, $1156 in the Philippines, $598 in Estonia and $745 in Tomsk. While the costs of treatment per patient differed greatly depending on the region, the cost per DALY averted was still below the per capita GDP in all four regions, making the interventions highly cost-effective. Even better cost effectiveness was achieved for outpatient care compared to hospitalisation. If the health effects observed for these regions are possible in other countries, an outpatient model of care would be recommended for treatment of MDR-TB. However despite the promising result, the quality of the data in the paper is limited, as none of the four studies were conducted in association with a randomised controlled trial and there was no comparison of different types of interventions within one region, as was the case for the 2006 Peru study. However, randomised controlled trial data are rarely used in economic evaluations, and the studies scored highly according to standard checklists for determining economic data quality.
Overall, the analyses suggest that treatment of MDR-TB using second-line drugs coupled with an individualised approach is highly cost-effective in low- and middle-income countries, particularly if the patients are not hospitalised. The challenge remains to implement such an intervention in all countries with a high burden of MDR-TB, but this can only be made possible with better detection rates. In the meantime, it is worth to investigate charities that work in this sector and make recommendations to donors based on the research.
References
- WHO: Media Centre
- WHO Global Tuberculosis Report 2013
- WHO: Biologicals
- WHO: Tuberculosis (TB)
- WHO: Tuberculosis (TB)
- WHO: Cost effectiveness and strategic planning (WHO-CHOICE)
- Giving What We Can Blog: Measuring benefits
- Baltussen R, Floyd K and Dye C. Cost Effectiveness Analysis of Strategies for Tuberculosis Control in Developing Countries. BMJ. 2005;331(7529):1364
- DCP2 Chapter 16
- WHO: Tuberculosis (TB)
- WHO: Tuberculosis
- Resch S, Salomon J, Murray M et al. Cost-Effectiveness of Treating Multidrug-Resistant Tuberculosis. PLOS Medicine. 2006;3(7):e241
- WHO: CHOosing Interventions that are Cost Effective (WHO-CHOICE)
- Fitzpatrick C and Floyd K. A Systematic Review of the Cost and Cost Effectiveness of Treatment for Multidrug-Resistant Tuberculosis. Pharmacoeconomics. 2012;30(1):63-80