Malaria
Giving What We Can no longer conducts our own research into charities and cause areas. Instead, we're relying on the work of organisations including J-PAL, GiveWell, and the Open Philanthropy Project, which are in a better position to provide more comprehensive research coverage.
These research reports represent our thinking as of late 2016, and much of the information will be relevant for making decisions about how to donate as effectively as possible. However we are not updating them and the information may therefore be out of date.
Cause Area: Malaria
According to the World Health Organization, in 2015 there were about 214 million cases of malaria. The disease caused 438,000 deaths, of which 306,000 were of children under 5.[^fn-1] However, the total number of deaths could be as high as one million1 since, due to diagnostic limitations, malaria incidence is hard to assess.2 About 3.2 billion people --- almost half the world’s population --- are at risk of malaria.3 Nintey-one percent of all malaria deaths occur in Sub-Saharan Africa4.
Symptoms of malaria include fever, headache, and vomiting, and usually appear between 10 and 15 days after the mosquito bite. Severe malaria may manifest in different ways, including: impaired consciousness, prostration, multiple convulsions, deep breathing and respiratory distress, acute pulmonary oedema and acute respiratory distress syndrome; circulatory collapse or shock.5 It can also lead to cerebral malaria, which can lead to serious neurological and cognitive deficits and is fatal for 15 to 25% of African children suffering of the disease.6 As of 2011, a minimum of 575,000 children in Africa developed cerebral malaria annually.7 Malaria is also extremely dangerous for pregnant woman: risks of malaria in pregnancy include maternal anemia, low birth weight (LBW), preterm delivery and increased infant and maternal mortality.8 Research also shows that malaria affects educational and economic outcomes.
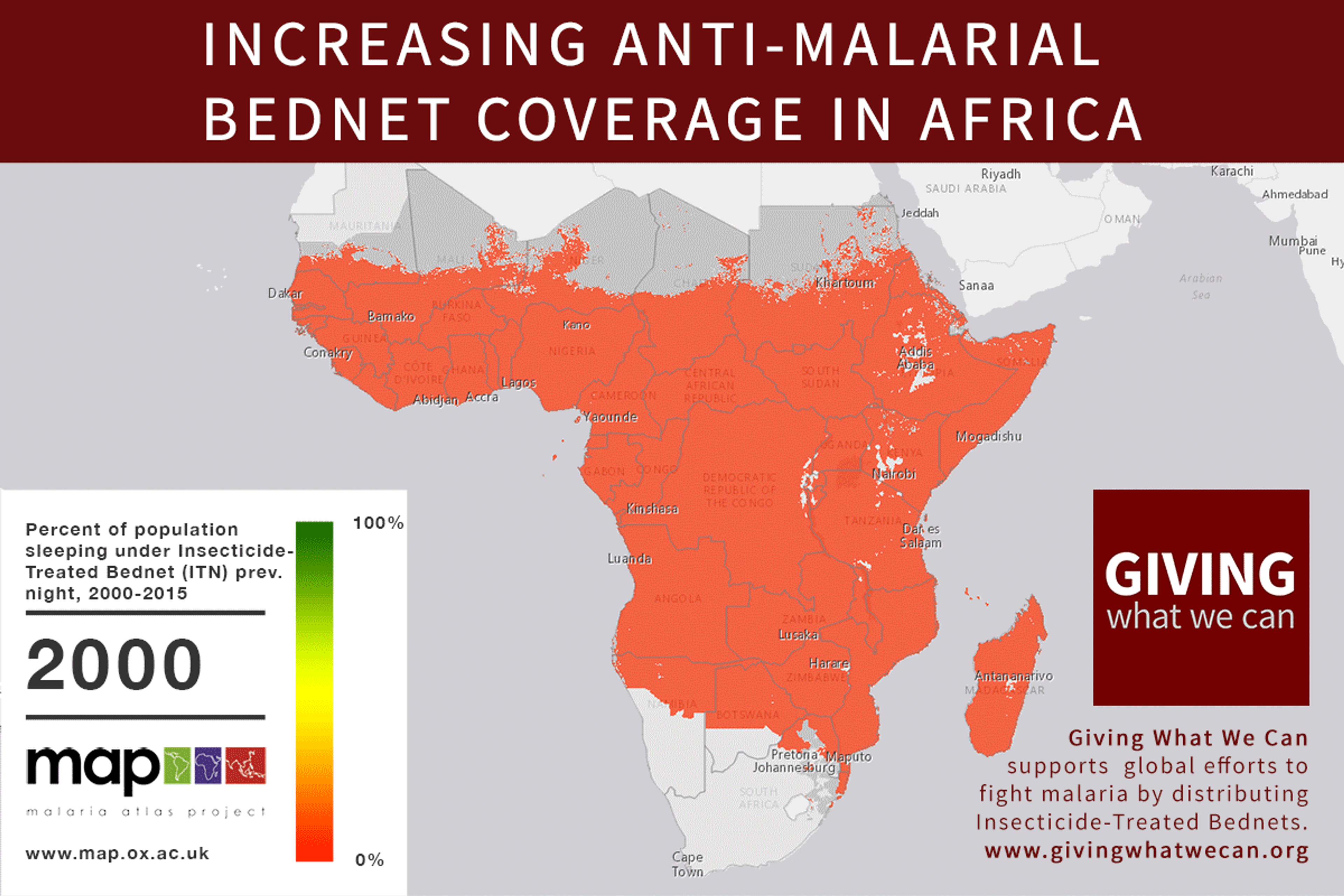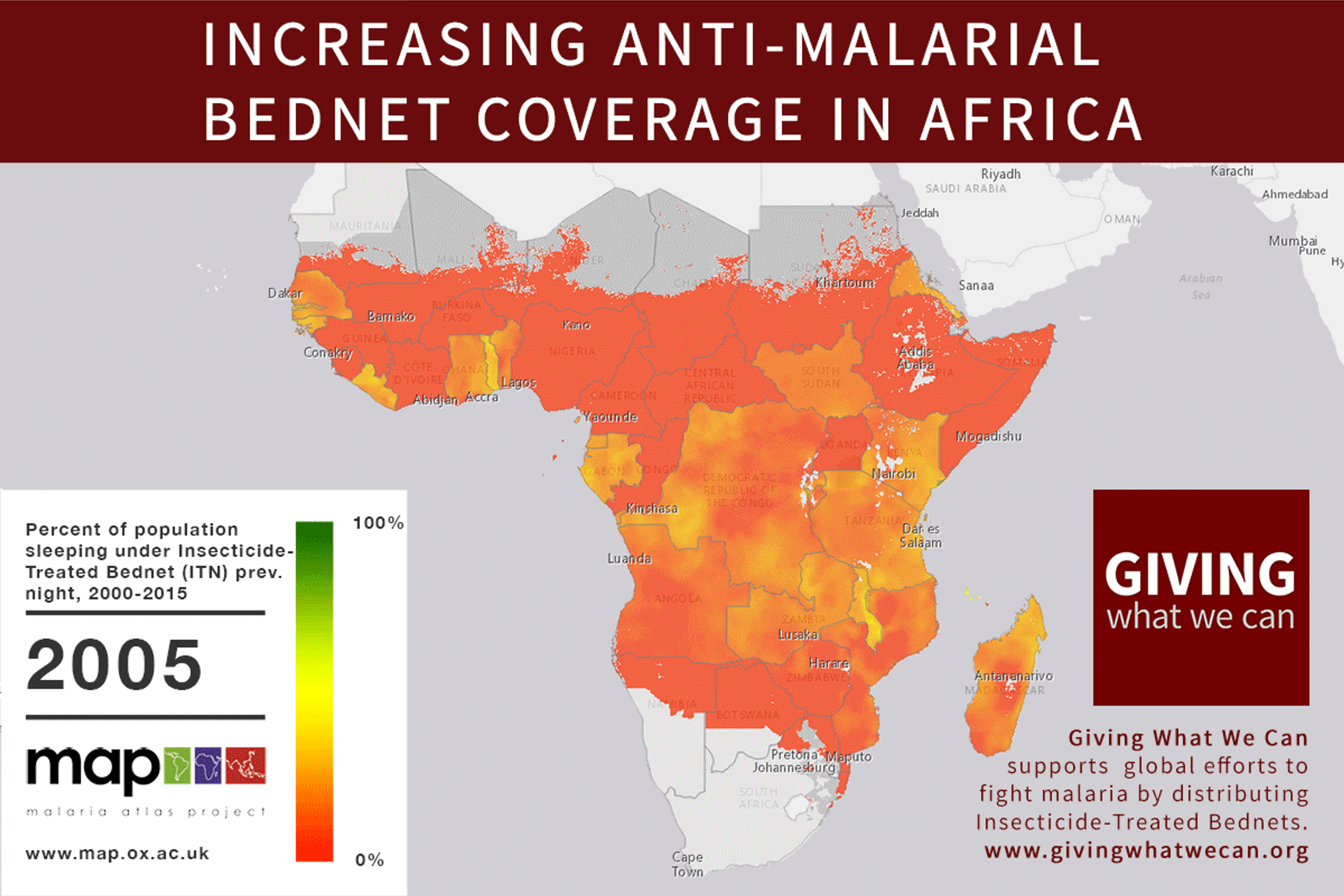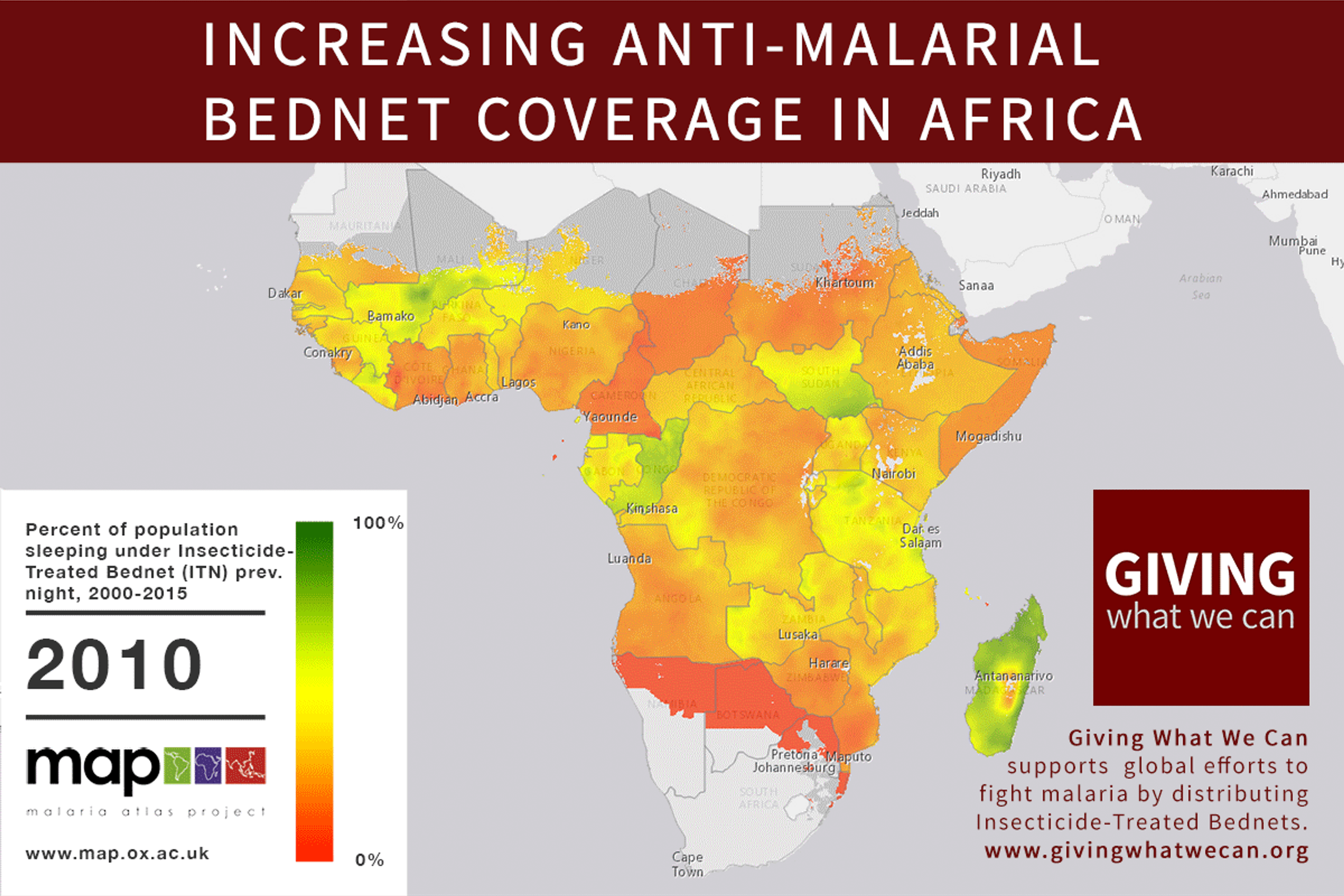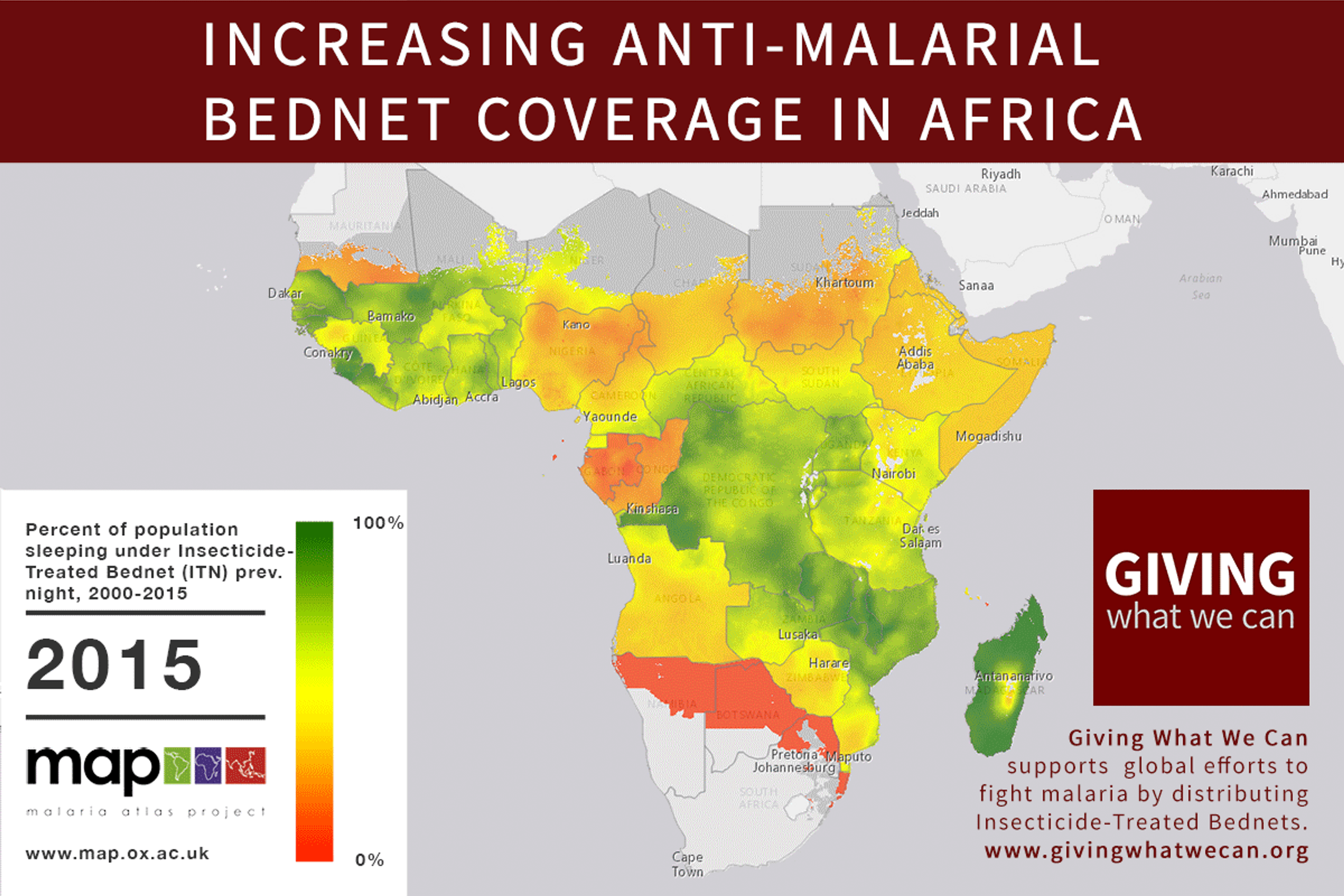
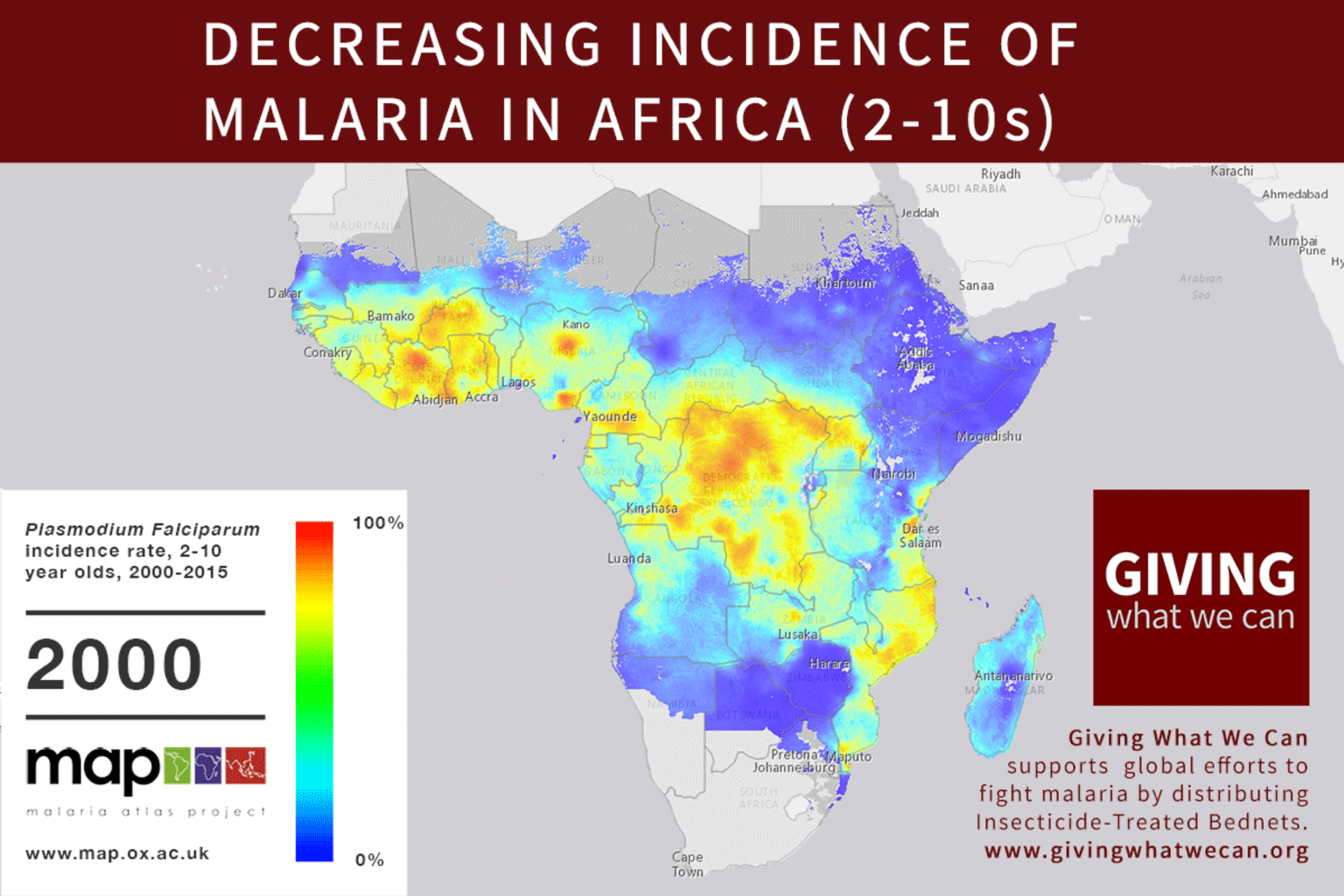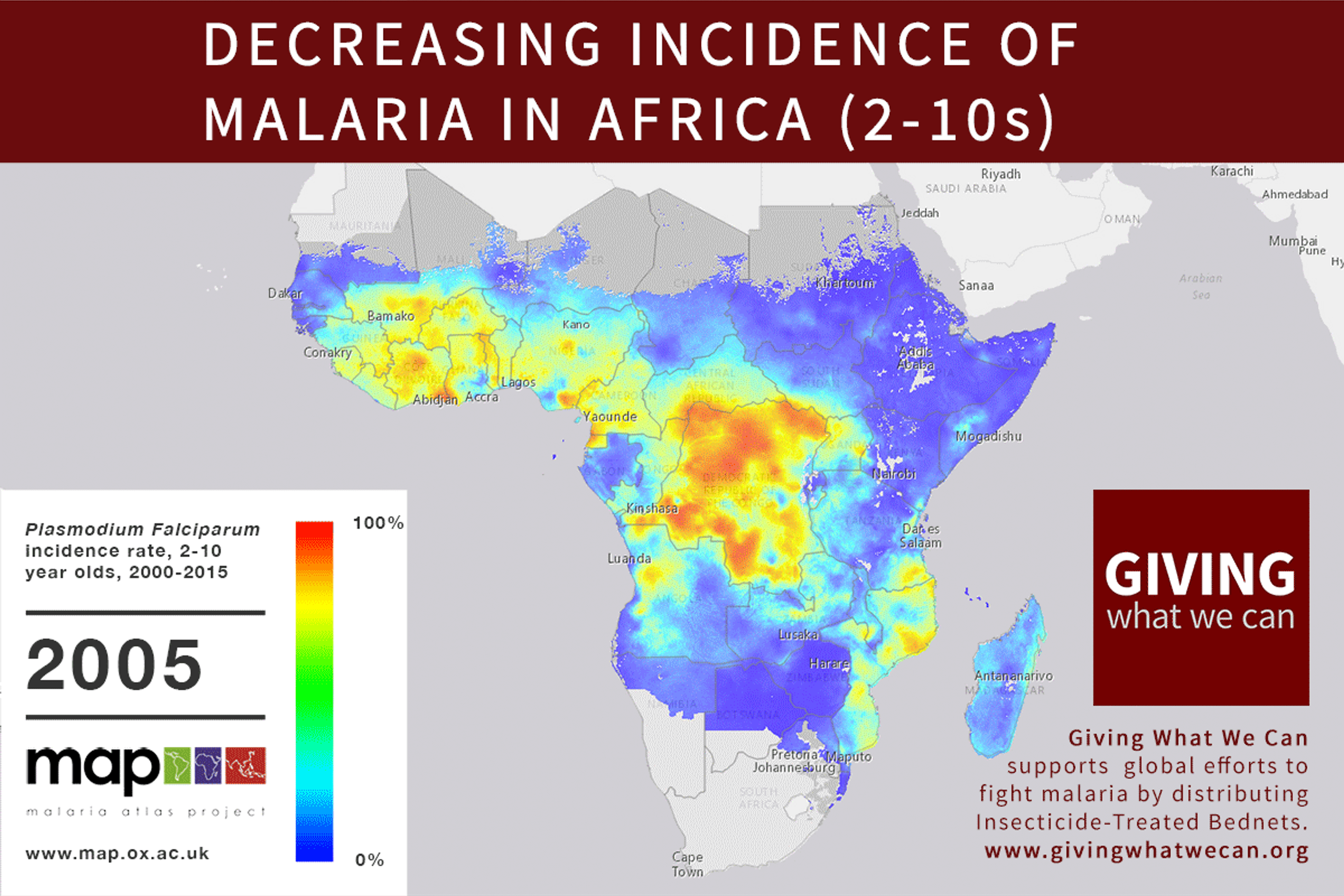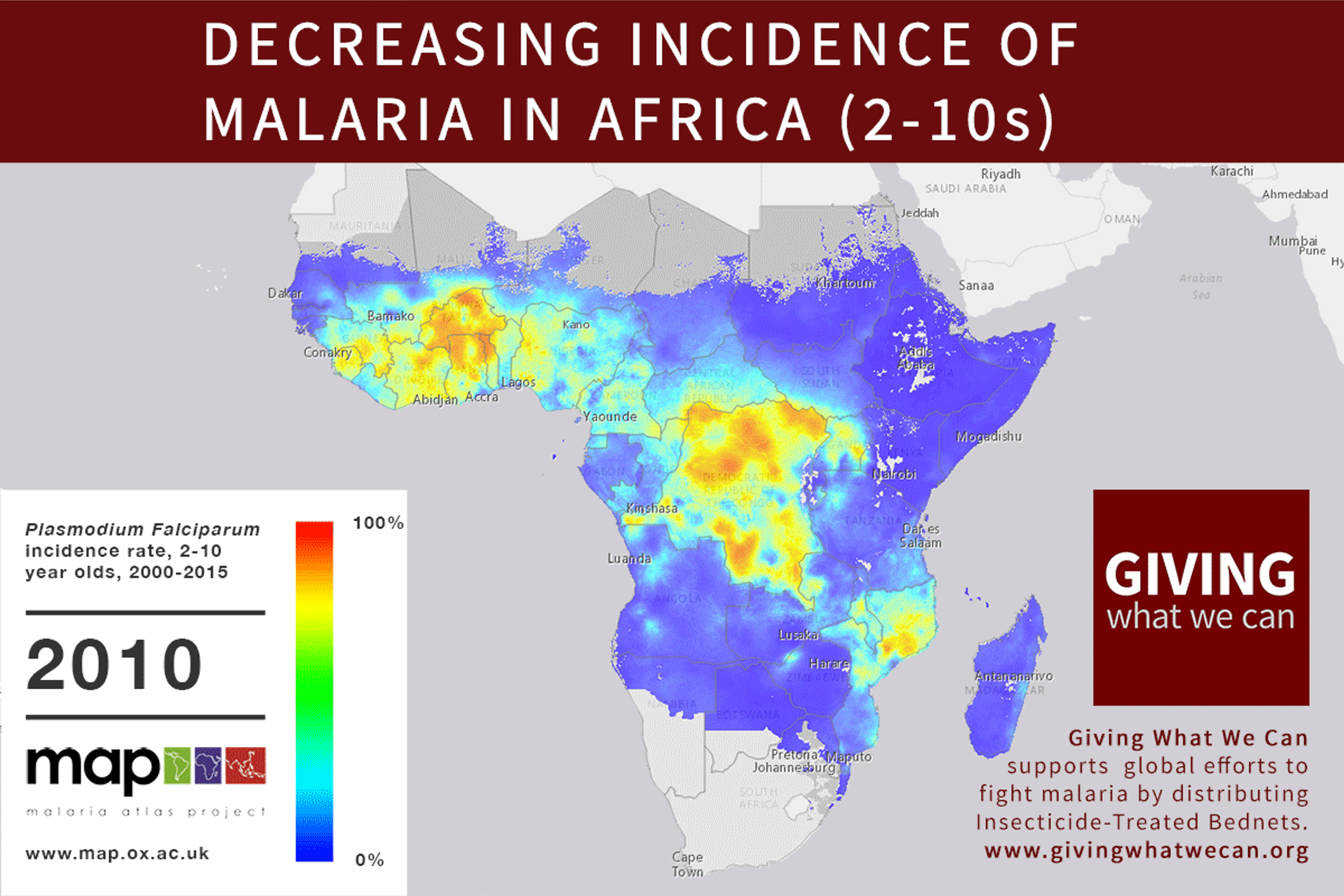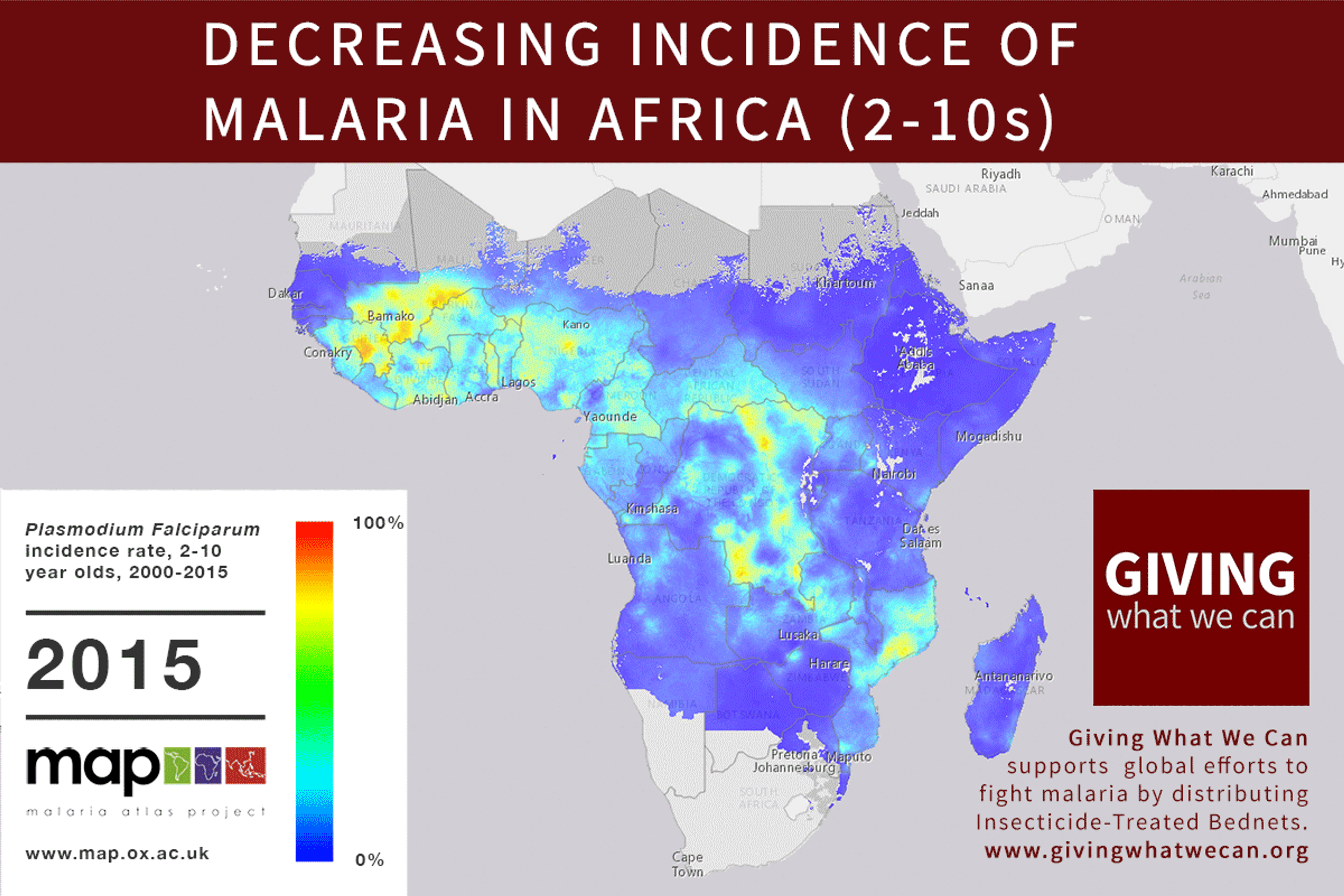
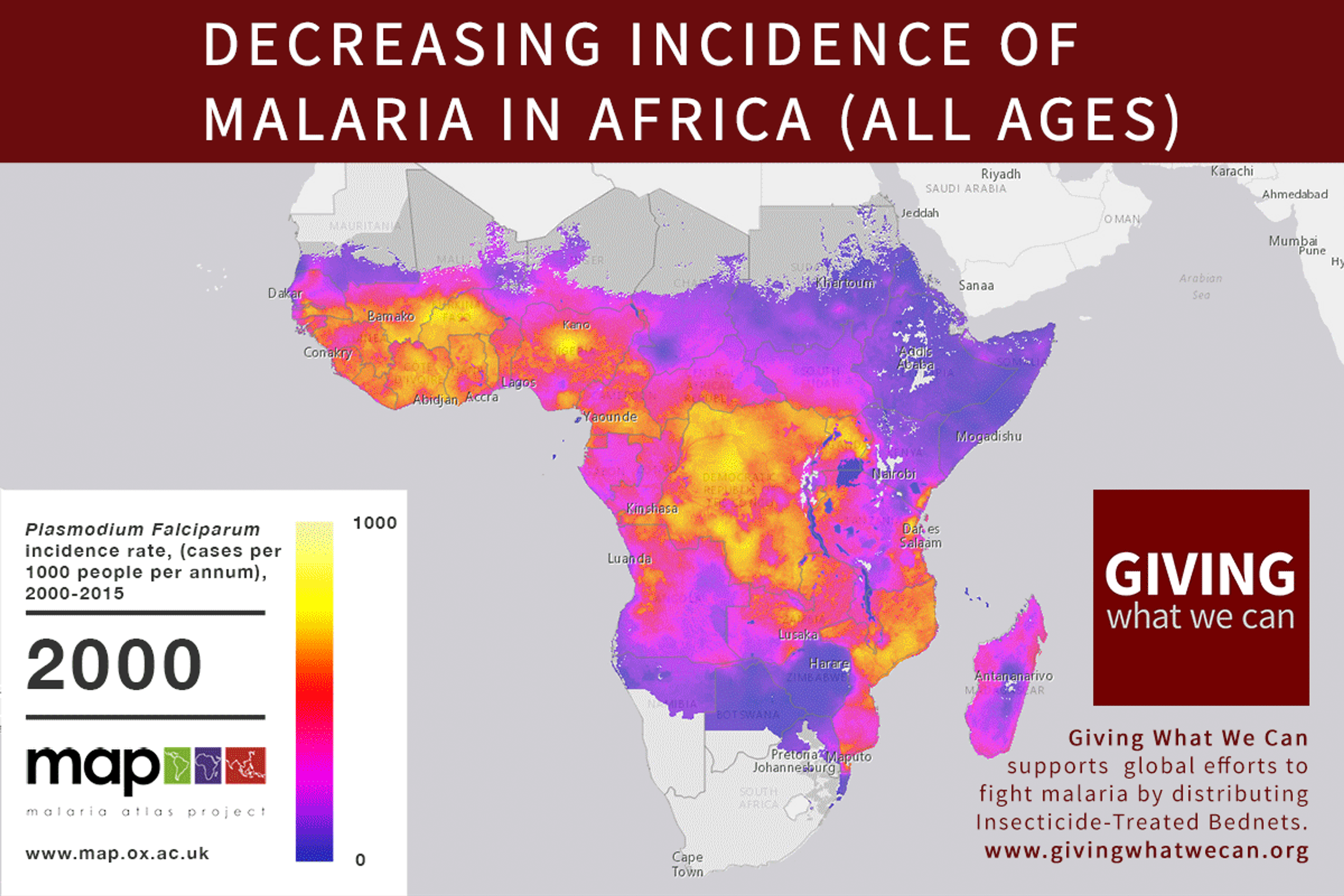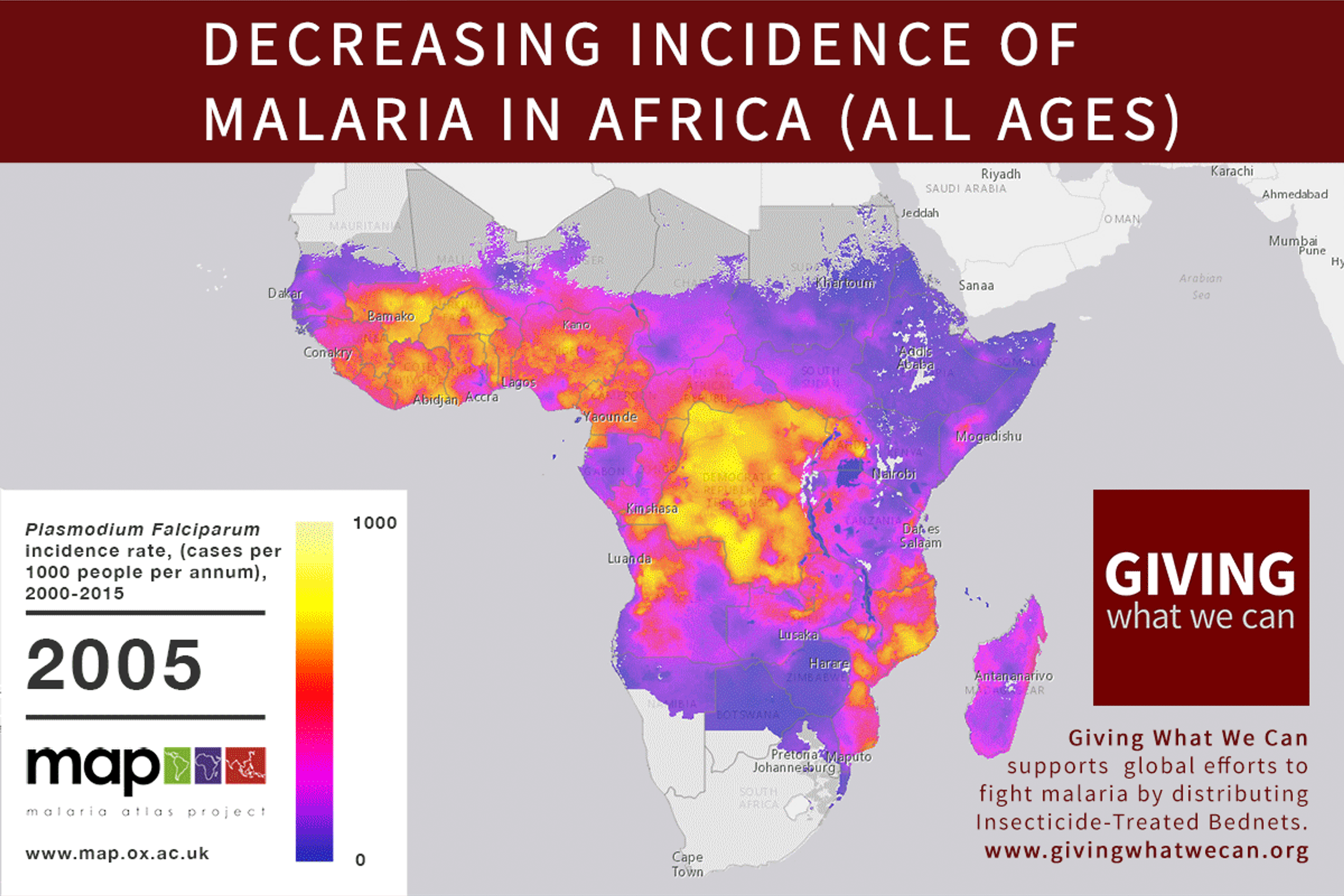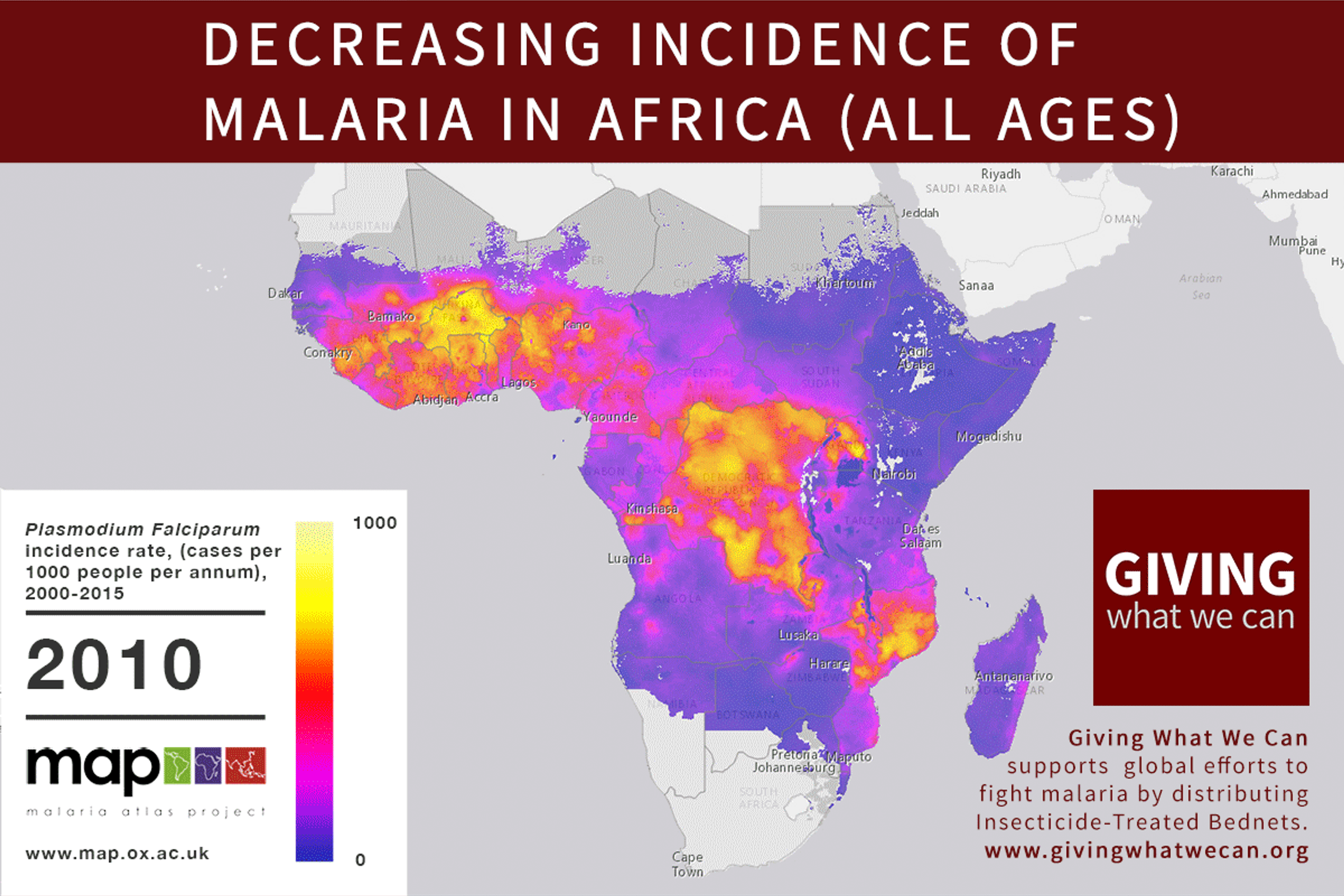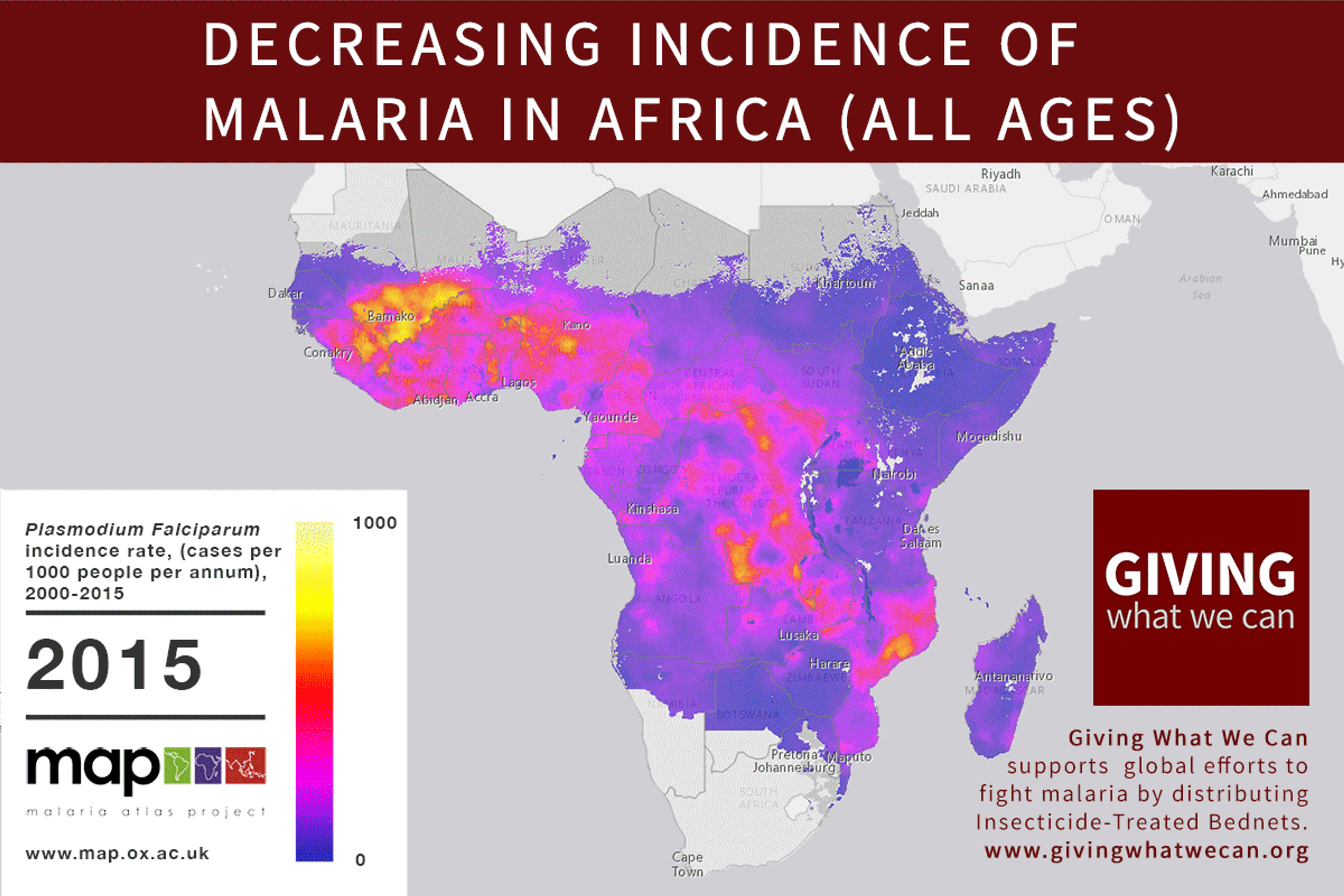
Long-lasting insecticide treated bed nets have prevented around 450 million cases of malaria in the last 15 years.9 The effectiveness of LLIN distribution in reducing malaria prevalence is very strong.10 Their role in reducing malaria morbidity and mortality has been shown for areas of both high and low endemicity.111213 A Cochrane meta-study, which consolidated high quality randomised controlled trials on LLINs, found that, for each 1,000 children protected with a net for a year, 5.53 deaths were averted. Extrapolating from these figures, GiveWell estimate that the cost per child life saved through an AMF-funded LLIN distribution at about $3,461 (this includes distribution and monitoring costs).14
We address some common concerns about negative and offsetting effects of bed nets distributions, including: insecticide resistance, possible negative effects of free bednet distribution on recipients’ usage rates and private markets, and whether bednets reduce malaria immunity. We also conclude that bednet distribution do not have a substantial disruptive effect on the wider economic and health system.
It is not easy to estimate the global gap in bed net provision, but evidence suggests that, at the minimum, in 2016 there is likely to be a gap of about 60 million nets (343 million dollars). However, the gap could be much larger, since estimates vary considerably: for instance, for 2015, the Roll Back Malaria estimated a gap of around 39 million nets and the WHO estimated a gap of about 146 million nets.
1. Importance
1.1 What is malaria?
Malaria is caused by a parasite called Plasmodium. The parasite is transmitted via the malaria-infected Anopheles mosquitoes. When the mosquitoes bite, the parasites are transported through the human body to the liver, where they multiply and infect red blood cells. Symptoms of malaria include fever, headache, and vomiting, and usually appear between 10 and 15 days after the mosquito bite. If untreated, malaria can quickly become life-threatening as it disrupts the blood supply to vital organs. The most deadly strain of malaria, P. falciparum is particularly prevalent in Africa.15 P. vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa.16
1.2 Morbidity and mortality
According to the World Health Organisation in 2015, there were about 214 million cases of malaria. The disease caused around 438,000 deaths, of which 306,000 were of children under five.17 91% of all malaria deaths occur in sub-Saharan Africa18. About 3.2 billion people --- almost half the world’s population --- are at risk of malaria.19
Due to diagnostic limitations, malaria incidence is hard to assess.20 A recent study21 used a more sensitive test for asymptomatic malaria than the one usually employed and showed that malaria is more common than previously thought: the prevalence of the disease in the study’s population had been underestimated by 8%. A review has recently concluded that the morbidity burden of the disease has been underestimated, in particular with regards to adult morbidity22. The total number of deaths could be as high as one million.23
In the developing world, malaria accounts for about 10% of all deaths of children under the age of five (see figure 1). If neonatal mortality is excluded, this figure rises to about 25%.24
Figure 1: Malaria makes up a large proportion of infant mortality in the developing world25
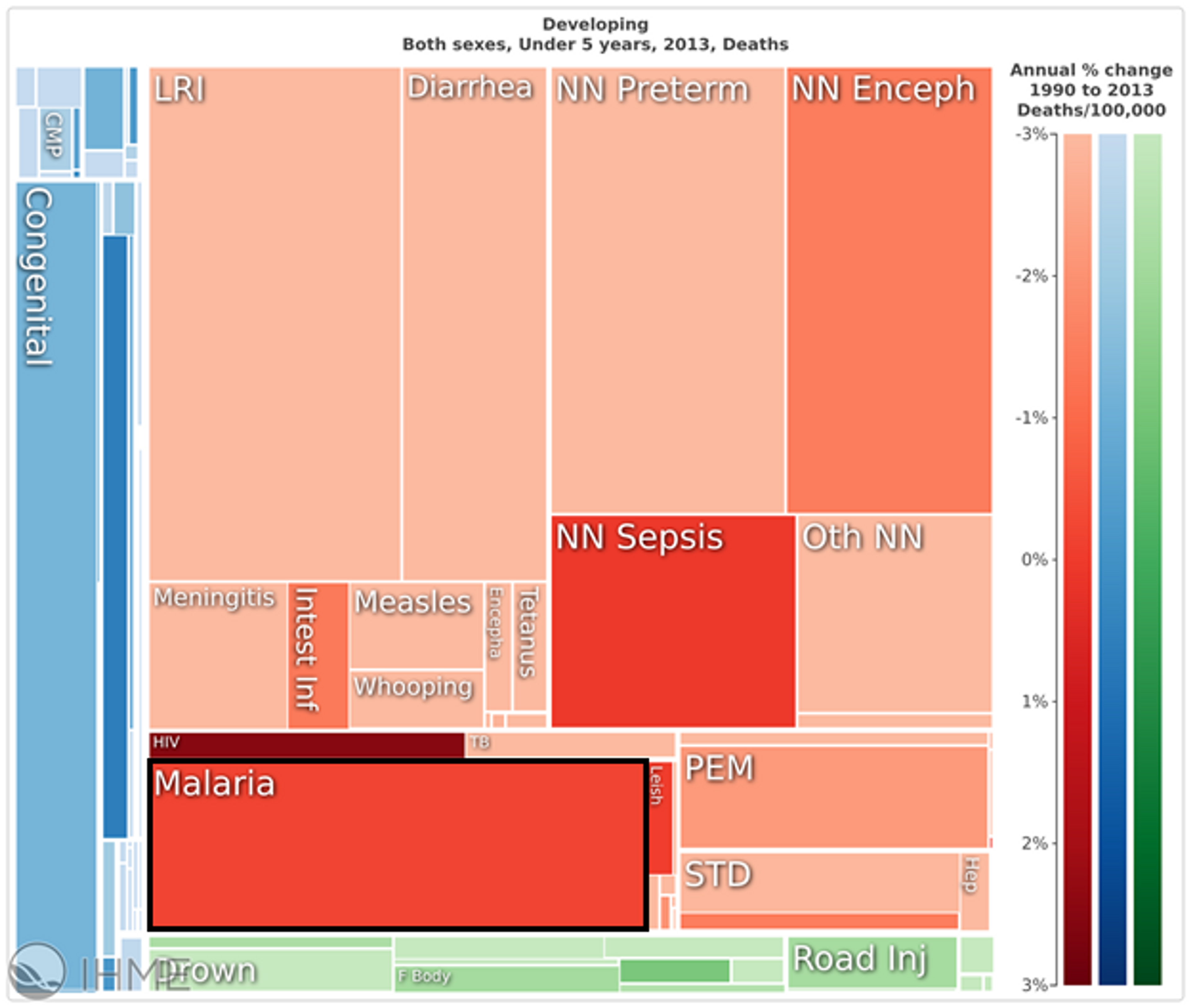
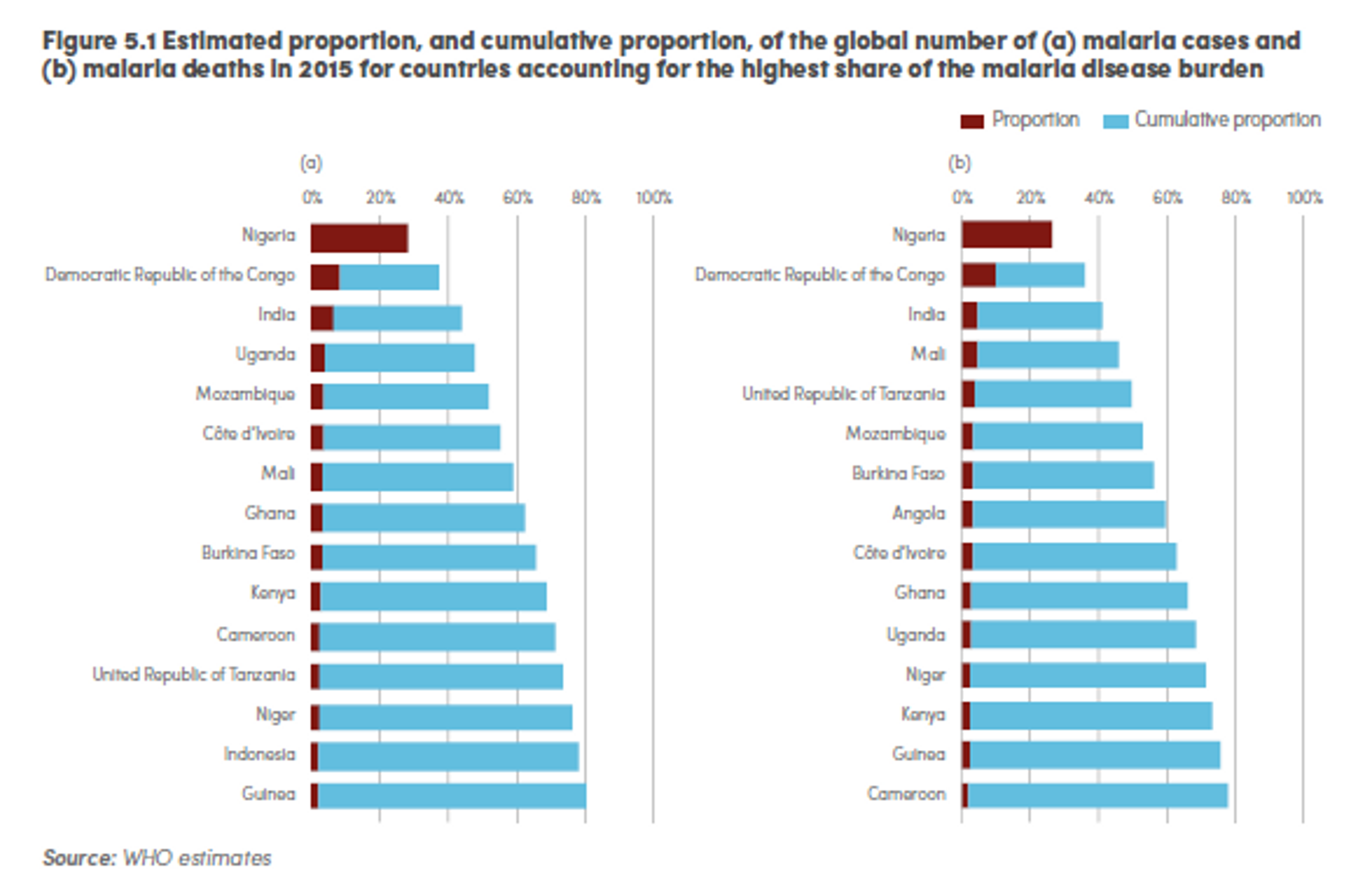
Most of the morbidity and mortality burden of the disease is highly concentrated: in 2015, it is estimated that 15 countries accounted for 80% of cases, and 15 countries accounted for 78% of deaths.26
Figure 2: Estimated proportion, and cumulative proportion, of the global number of (a) malaria cases and (b) malaria deaths in 2015 for countries accounting for the highest share of the malaria disease burden27
Severe malaria may manifest as impaired consciousness, prostration, multiple convulsions, deep breathing and respiratory distress, acute pulmonary oedema and acute respiratory distress syndrome; circulatory collapse or shock, systolic blood pressure, acute kidney injury; clinical jaundice plus evidence of other vital organ dysfunction; and abnormal bleeding.28
Severe malaria can lead to cerebral malaria, which causes abnormal behavior, impairment of consciousness, seizures, coma, or other neurologic abnormalities.29 It mainly affects pre-school children and, as of 2011, a minimum of 575,000 children in Africa developed cerebral malaria annually.30 Case fatality rates among African children with cerebral malaria are in the range of 15 to 25%.31 Cerebral malaria can lead to serious neurological and cognitive deficits, which we discuss below.
Malaria is also extremely dangerous for pregnant woman: risks of malaria in pregnancy include maternal anaemia, low birth weight (LBW), preterm delivery and increased infant and maternal mortality.32
1.3 Malaria as a risk factor for other conditions
There is strong evidence linking malaria with anemia,33 low birthweight,34 and neurological problems.35 More recent studies have also suggested a link with HIV36 and high blood pressure in children,37 although this is more uncertain. A recent article argues that malaria has serious consequences also in absence of symptoms such as fever and chills. These include increasing the risks of anemia, maternal and neonatal mortality, bacterial co-infection, and cognitive impairment.38
Malaria and anemia
Malaria has been linked with anemia in vulnerable groups, such as pregnant women, young children and HIV patients. Meta-analyses of intervention trials suggest that successful prevention of malaria infections reduces the risk of severe maternal anaemia by 38%39 and a recent paper showed a significant association between anemia and asymptomatic malaria among pregnant women40. Observational epidemiologic studies have also demonstrated that malaria is an important correlate of anemia in young children. These findings suggest that global health strategies to reduce the burden of anemia should prioritize malaria prevention41. Another affected group are HIV positive patients: a recent study found that almost all the HIV patients in a sample with malaria infection were anemic42. Few studies have looked at the effect of malaria on anemia in non-pregnant, non HIV-infected adults. However, one study in Cameroon suggested that in adult patients with fever, malaria parasitaemia contributes to anaemia43.
Malaria and maternal and newborn health
There is strong evidence suggesting malaria affects neonatal and maternal health, increasing chances of low birth weight and perinatal mortality. A 2007 Lancet article argues that successful prevention of malaria infections reduces the risk of low birth weight by 43%, and perinatal mortality by 27% among paucigravidae.44 The evidence shows that sleeping under nets increased mean birth weight by 55g, reduced low birth weight by 23%, and reduced miscarriages/stillbirths by 33% in the first few pregnancies. Another more recent large scale observational study found that first-trimester falciparum and vivax malaria both increase the risk of miscarriage.45 A study from Liberia found that one percent increase in maternal infection risk lead to 044 percent increase in one-year mortality.46 This strongly suggests the need for pregnant women to sleep under nets in Africa.47 However, as of 2010, insecticide-treated nets were used during pregnancy for only 10.5 million of 26.9 million births across 37 countries.48
Malaria and neurological disorders
Evidence suggests that malaria leads to neurologic and cognitive deficits, and an increased risk of behavioural disorders.49505152535455 Cerebral malaria has also been associated with epilepsy. A recent meta-analysis showed that survivors of cerebral malaria are five times more likely to suffer from epilepsy.5657 We place weight in this meta-analysis as it controlled for publication bias and had a clear criteria for study inclusion. As such, we believe that, as preventative measures to reduce malaria are very cost-effective, donating to a charity in this area represents a good opportunity to reduce the burden of mental health disorders in the developing world. Our model suggests that the distribution of long-lasting insecticide treated bednets averts one case of epilepsy for about $25,000. This is comparable to the most cost-effective interventions to treat epilepsy and ignores all other additional benefits.
Malaria and HIV
There is some evidence that malaria increases HIV transmission.58 In 2006, a paper published in Science showed that people who are HIV positive have shown a spike in HIV viral load during a malaria fever episode.59 They found that, a malaria fever can increase HIV viral load by almost one log (10 times) --- and stay at an increased level for a duration of up to six weeks.60 A recent systematic review and meta-analysis found that acute malaria increases HIV viral load by only 0·67 log10.61
According to a recent review, the higher viral load increases the risk of transmitting HIV in communities with high HIV prevalence, though it has no effect in areas with low HIV prevalence.62 Another study showed that individuals who live in areas with high malaria parasite rate have about twice the risk of being HIV positive compared with individuals who live in areas with low malaria parasite rate, after controlling for important socioeconomic and biological factors.63
Some researchers have suggested that it is high HIV viral loads in Sub-Saharan Africa, partly because of high rates of coinfection, may have been one of the drivers of the “explosive” epidemics seen in that region.64 However, a very recent study65 modelling the coinfection of HIV with malaria and other diseases such as schistosomiasis argues against this thesis, on the grounds the duration of coinfection is too short and/or the viral load elevation is too modest to explain the epidemics.
Independently of whether and by how much HIV transmission risk is increased, coinfections might have implications for AIDS progression. One study suggested that people with HIV that were provided with bednets had slower progression to AIDS.66 Another study showed that people with HIV who were provided nets and water filters delayed initiation of antiretroviral therapy, potentially due to this slower progression to AIDS.67 This study estimated this intervention to be highly cost effective: when combining the benefits due delayed initiation of antiretroviral therapy with other health benefits due to fewer malaria cases, this intervention costs $99 USD per disability-adjusted life year (DALY) averted (with net cost savings for the health system). However, a recent review concluded the data regarding the impact of net provision and/or use on malaria morbidity reduction in people with HIV were limited.68
In sum, more research needs to be done to investigate this interaction, but there is good reason to think that malaria prevention can help limit HIV infection, even though it is difficult to estimate by how much.
Malaria and blood pressure
Research suggests that malaria during pregnancy might affect blood pressure in children and that this might may contribute to the African burden of hypertension, which is higher than in developed countries.69 However, it is unclear whether this effect is causal or merely correlational at this point.70
Malaria and cancer
Burkitt’s lymphoma is the fastest growing human tumour. The annual incidence has been estimated at 40–50 per million children younger than 18 years. The disease is associated with Epstein-Barr virus. Chronic infection with Plasmodium falciparum has been epidemiologically associated with endemic Burkitt’s lymphoma for over 50 years.71 The distribution of endemic Burkitt’s lymphoma across Africa and Papua New Guinea corresponds to areas of holoendemic malaria72 and the early acquisition of Epstein-Barr virus. In these high-risk areas endemic Burkitt’s lymphoma comprises about half of all childhood cancer diagnoses and up to 90% of lymphoma diagnoses.73
Malaria and kidney injury
Acute renal injury occurs when kidneys abruptly lose their functionality. Following World Health Organization criteria of the disease, acute renal failure occurs as a complication of Plasmodium falciparum malaria in less than 1% of cases, but the mortality rate in these cases may be up to 45%.74
Malaria and childhood stunting
A systematic review of observational studies found that most studies find no association between malaria and subsequent malnutrition in Plasmodium falciparum areas, but in Plasmodium vivax endemic areas malaria was associated with malnutrition in children.75 However, one study provides evidence that malaria episodes strongly increased risk for childhood stunting.76
1.4 Malaria’s effects on educational outcomes
Recent studies have highlighted that anti-malaria programs have a positive effect on educational outcomes, and might in fact be cost-effective educational interventions. A recent study examined the effects of the Global Fund’s malaria control programs on the educational benefits to primary schoolchildren in Sub-Saharan Africa.77 Using a quasi-experimental approach, they found that in 14 of 22 countries, the program led to substantial increases in years of schooling and grade level as well as reductions in schooling delay. Another study examined whether the Roll Back Malaria campaigns affected the educational attainment of primary schoolchildren across 14 countries in Sub-Saharan Africa. It found that the campaign substantially improved schooling attainment in 13 of 14 countries, at an average cost of $13.19 per additional year, which is highly cost-effective as compared to standard educational interventions.78
Various other studies also support the causal link between malaria eradication and improvement in educational outcomes using natural and quasi experiments, by connecting malaria eradication with improved years of schooling, literacy, and primary school completion,79 lifetime female educational attainment literacy,80 schooling rates for both adults and children,81 increases in Raven Progressive Matrices test scores (measuring abstract thinking skills),82 cognitive abilities and school performance.83 These results are plausible due to malaria’s affecting school absenteeism and cognitive function.84
1.5 The economic effects of malaria85
There is some evidence suggesting that people exposed to malaria earn substantially less over their lifetime.8687 One influential study reported a 25% increase in future earnings for areas in which malaria was eradicated in Latin America and a 12% increase in the U.S.88 These are very substantial results (the increases apply to the whole population rather than just those infected with malaria) but have limited external validity because the data examined were from Latin America and the U.S. (where the predominant strain of malaria differs substantially in its effects to the predominant strain of malaria in Sub-Saharan Africa), and the 1920s in the U.S. and 1950s in Latin America (when the economic ecosystem was very different).
However, more support to the thesis that malaria affects household earnings comes from more recent studies. A 2010 study found that malaria eradication in India lead to modest increases in household incomes for prime age men, though lead to no increase in education.89. A more recent study in Uganda estimated the gains at between 3% and 11%90 The paper is important for two reasons. First, it has greater external validity in Sub Saharan Africa. Second, because the prevalent strain of malaria in Uganda has a higher mortality rate, the results include the countervailing selection effect of removing lower lifetime income people from the population. The study found that the long term impact of malaria prevention on productivity is substantial, even net of this selection effect. The paper thus lends credence to the belief that malaria eradication could have long term productivity effects although we would welcome further research in this area.
The most direct economic benefit to reduced malaria prevalence is the associated reduction in household health expenditures. Getting malaria is not only potentially deadly but it’s also very expensive. This cost is relatively easy to measure. A review found that malaria treatment can account for a significant proportion of low income households’ expenditures.919293 In Malawi, malaria treatment accounts for 2% of monthly income for the average household, and 28% for poor households.94 In Kenya, malaria accounts for 7.2 percent of household expenditure on average in wet seasons and 5.9 percent in dry seasons; however, for households in the bottom quintile the ratios increase to 11 percent in wet seasons and 16.1 percent in dry seasons.95
Where healthcare costs are subsidised, the direct costs of treating malaria fall on the government. The 2015 WHO World Malaria report estimates that, since 2000, 263 million cases of malaria which would have been treated in the public sector have been averted. This is equivalent to a $900 million saving for government budgets, of which $610 million is attributable to distribution of ITNs/LLINs.96 The 2011 version of the same report noted that “in Rwanda it has been estimated that while it would cost $265 million USD to sustain the malaria control programme over the next five years, the public health system could avert about $267 million USD in the costs of diagnosing and treating malaria; and households could avert about US$ 547 million in direct and indirect costs, equivalent to about 7% of household income.”9798
The economic cost of malaria is not limited to the direct costs of treatment. Working when you are seriously ill is very difficult. As a result, the sick take time off work (or at least are less productive) and those with sick children take time off to care for them. Time lost per episode varies significantly across settings due to the variability of different types of malaria strains, type of economic activity and access to treatment amongst other factors. The average time lost per adult varies between 1 and 5 days.99100 One recent RCT found that a group of farmers who were assigned bednets increased their harvest value by 15%101 (although Givewell have questioned the validity of this result due to baseline imbalances between the treatment and control groups). However, there remains considerable debate as to how much malaria impacts short term productivity. For example, in Malawi, only 52% of adults reported that their illness affected their work102. Moreover, a larger RCT which looked at 81,597 smallholder contract farmers in 1,507 clusters found no significant impact on cotton production from bednet distribution.103 One possible explanation for this result is that when people are ill they deprioritise less lucrative activities such as basket weaving rather than cotton farming. It seems likely that people who have malaria are less productive in the short term but the scale of this impact is highly uncertain.
Macroeconomic impact of health improvements is particularly difficult to measure. Early attempts used simple country cross-sectional regressions of health on GDP per capita104105 but these seem likely to be methodologically unsound because of omitted variable bias and the causal circularity of health and income (improved health may lead to increased income but increased income also leads to improved health). As a result, much of the literature prefers to extrapolate overall macroeconomic impact from microeconomic productivity gains.106107
More promising is the use of time-series data and simulations to evaluate the impact of a health shock on income per capita. Ashraf et al. simulated the impact of the immediate eradication of two diseases, malaria and tuberculosis.108 They found that, while the eradication of tuberculosis may lead to immediate increases in income per capita, the eradication of malaria may lead to short term reductions in income per capita. The difference is explained by the different demographic effects of the two diseases. While tuberculosis primarily affects workers in their prime, malaria disproportionately kills children under the age of 5. Malarial eradication therefore increases the dependency ratio of children to workers in the short term, leading to a fall in income per capita. In the long term, declining endogenous fertility rates, combined with the productivity improvements discussed above mean income per capita rises again (see Figure 3).
Figure 3: Simulated impact of elimination of malaria and tuberculosis on income per capita109
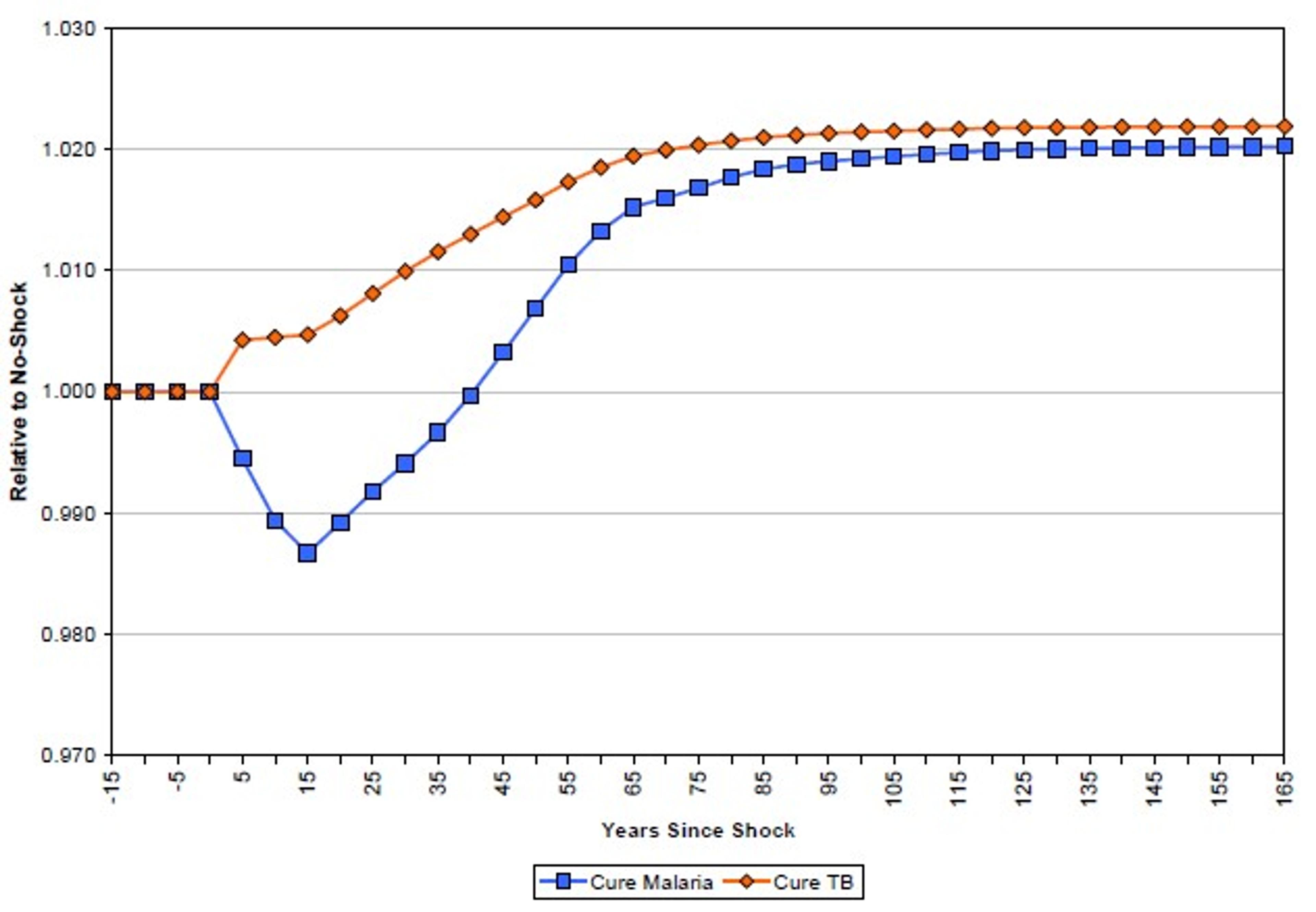
These results have been thoughtfully critiqued by Bleakley.110 While Bleakley agrees that demographic effects mean the impact of malaria eradication is likely to be realised in the long term rather than the short term, he notes, among other things, that the mechanisms through which malaria impacts productivity may not be limited to cases of acute fever (which is assumed by Ashraf et al.) but rather may have more insidious effects on long term productivity. Bleakley concludes that Ashraf’s estimates may be an order of magnitude too low. The debate seems to hinge on the scale of these long term productivity effects, which are discussed above.
2. Tractability
2.1. How can the problem be addressed?
Malaria control is carried out through a combination of interventions such as vector control, chemoprevention and case management. Vector control consists of preventing mosquitoes from infecting people and acquiring the infection. The two main ways to achieve this goal are insecticide treated mosquito nets (ITNs) and indoor residual spraying (IRS). Case management consists in process including detection, diagnosis, treatment and cure of the infection. Chemoprevention prevents the infection from developing in people by administering antimalarial drugs, and it is especially used for pregnant women and children.111
_Figure 4: Main strategies to prevent and treat malaria112
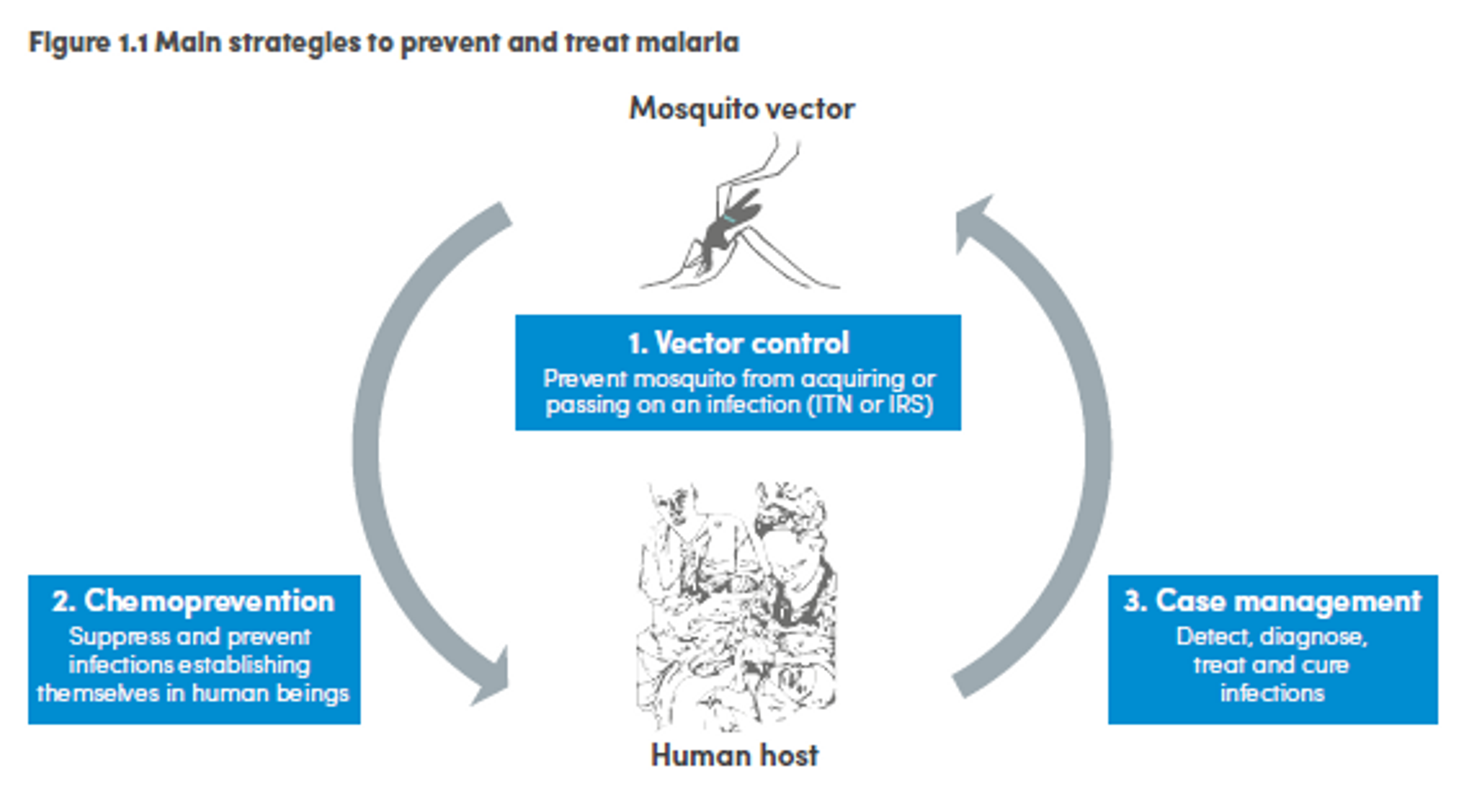
Of these, it seems that insecticide-treated bednets (ITNs) are the best opportunity for donors due to their cost-effectiveness, and strong track record of achieving results. Recent research suggests that anti-malarial interventions have prevented about 663 million malarial fevers from 2000–2015. Globally, 6.2 million fewer people died of malaria over the last 15 years because of malaria interventions.113 Long-lasting insecticide treated bed nets stand out as being particularly effective --- being responsible for around 68% of the malaria reduction. This means that bednets have prevented around 450 million cases of malaria.114
Other strategies
Indoor residual spraying
Aside from bednet distribution, vector control can be achieved by indoor residual spraying. The WHO recommends (as well as universal coverage with LLINs of at-risk population), targeted indoor residual spraying for the control and elimination of malaria.115 Evidence shows IRS is effective in reducing malaria transmission116 and recent estimate suggests IRS averted 6.8 million clinical cases of malaria in Africa since 2000 (10% of the cases averted overall).117
Mass drug administration
Another tool in the fight against malaria that has attracted attention in the last years is mass drug administration (MDA) of ivermectin. Ivermectin can kill biting malaria vectors and lead to reduction in the parasitetransmission118119. A recent review shows that single ivermectin MDAs is associated with a significant reduction in malaria transmission120 and another study argues that ivermectin MDAs should be seriously considered as a new tool against malaria, since nets do little to prevent outdoor transmission.121 However, it should be stressed that ivermectin MDAs is not presented as a replacement to bednet distribution, but rather an additional tool in the fight against the disease.
Vaccines
Recently, a new vaccine, Mosquirix, has passed Phase III trials and was recommended by European drugs regulators.122 A 2015 analysis based on mathematical modelling estimates that, depending on the area of implementation, the vaccine could potentially prevent 6%–30% of deaths in children younger than 5 years, when added to existing coverage of long-lasting insecticide-treated nets and with moderate levels of malaria treatment. Mosquirix is expected to be rolled out in 2017.123 It is important to note that the vaccine is only 35% effective, that it is likely not much more or even less cost-effective than bednets,124 and that experts agree that it should be used in combination with bednets.125 Thus, we believe the new vaccine is not a replacement for bednets nor should it be prioritized for the time being.
Gene drives
Much attention has recently been received by the development of a new technique for malaria prevention through genetically modifying mosquitoes. For a while, scientists have been able to isolate genes that could confer resistance to P. falciparum.126 Recently, significant progress has been made in developing “gene drives,” which is a way to ensure these genes are transmitted to the wider mosquitoes population.127128 However, gene drives have the potential for altering entire ecosystems, and are thus a controversial technique: safety nets would thus be needed before it could be implemented in the field.129
2.2 Distribution of long-lasting insecticide treated bednets
An insecticide treated net (ITN) is a net, usually intended to be hung over the bed at night. As the mosquitoes responsible for spreading the parasite usually feed at night, this provides a high level of protection against infection. ITNs are treated with insecticides which kill mosquitos and are estimated to be twice as effective as untreated nets at reducing infections.130 Long-lasting insecticide treated nets (LLINs) are nets designed to remain effective for longer periods without retreatment.
Distribution of LLINs is undertaken by Ministries of Health, NGOs, and distribution partners. Activities include a pre-distribution survey to assess need, delivery of LLINs, promotion of the use of LLINs, and post-distribution surveys to monitor usage. In particular, the WHO stresses that behaviour change communication strategies are needed to ensure that nets are properly maintained and used.131
Bednets’ effect on malaria morbidity and mortality
The evidence for the effectiveness of LLIN distribution in reducing malaria prevalence is very strong.132 Their role in reducing malaria morbidity and mortality has been shown for areas of both high and low endemicity.133134135 A Cochrane meta-study, which consolidated 22 high quality randomised controlled trials on LLINs, found that, for each 1,000 children protected with a net for a year, 5.53 deaths were averted. This result was broadly consistent across a range of different settings.136 The author of this study has suggested that further RCTs on ITN effectiveness would be unethical, as it would be denying the control group a treatment which is known to be beneficial.137
Cost effectiveness of bednet distributions
Cost per death averted
Extrapolating from these figures above, GiveWell estimates that it would cost $3,461 to save a life (this includes distribution and monitoring costs) through interventions Against Malaria Foundation is part of.138 This suggests that LLIN distributions are one of the most cost-effective public health interventions available to donors.
How does this figure align with other estimates in the literature? Cost–benefit analysis of ITN delivery in Kenya between 2003 and 2008 suggests that the cost per infant death averted by ITNs was $1011.87.139 A model called "Lives Saved Tool" was used to quantify the likely impact that malaria prevention intervention scale-up has had on malaria mortality over the past decade (2001-2010) across 43 malaria endemic countries in Sub-Saharan Africa. The model estimated that the scale-up resulted in $2,770 per life saved.140 A 2011 article systematically reviewed the published literature on the costs and cost effectiveness of malaria interventions. The graph below shows shows the incremental cost effectiveness ratio (ICER) of anti-malarial interventions against deaths averted, DALYs averted and cases of malaria averted.141 ICER is defined by the difference in cost between two possible interventions, divided by the difference in their effect.
Figure 5: ICERs of anti-malarial interventions against deaths averted, DALYs averted and cases of malaria averted 142
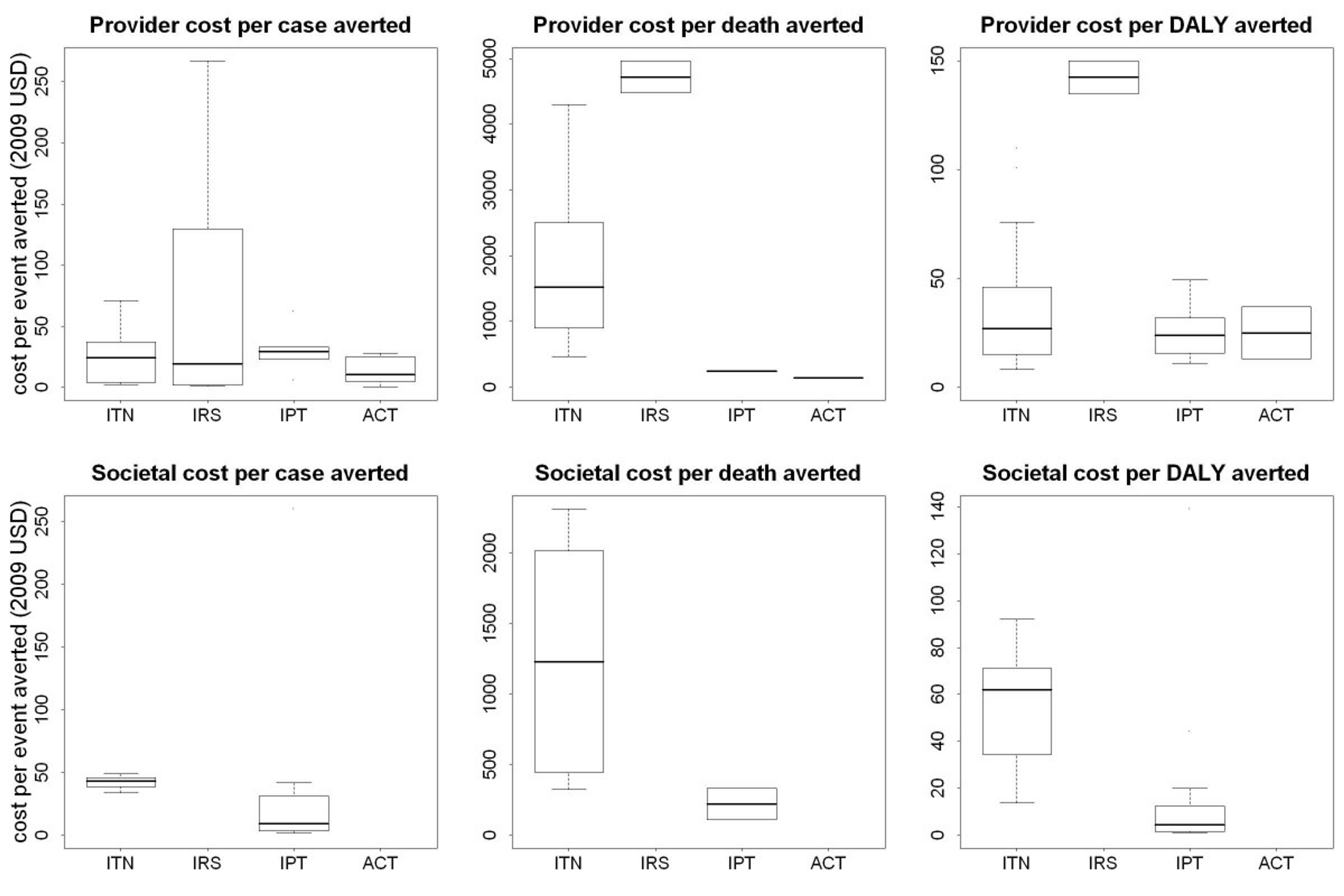
These figures are similar to the one published in a recent Lancet article,143 which estimates that, in low-income countries, it costs $4,205 to save a child’s life. They are also comparable to the figure that health economists estimate it will cost in the coming years to prevent a death in low income countries from 2015-2030: $4-11k per death prevented.144 For comparison, a recent study estimated the "Value of a Statistical Life," i.e., what people are willing to spend to prevent a death in the United States, based on what is spent on airbags. The study suggests that between $7 million and $13 million is spent to prevent one death.145
Cost per DALY averted
A simulation of the cost effectiveness of different interventions in Rachuonyo South District, western Kenya, lead to the following results:146
Figure 6: Simulation of the cost-effectiveness of different interventions in Rachuonyo South District, western Kenya 147
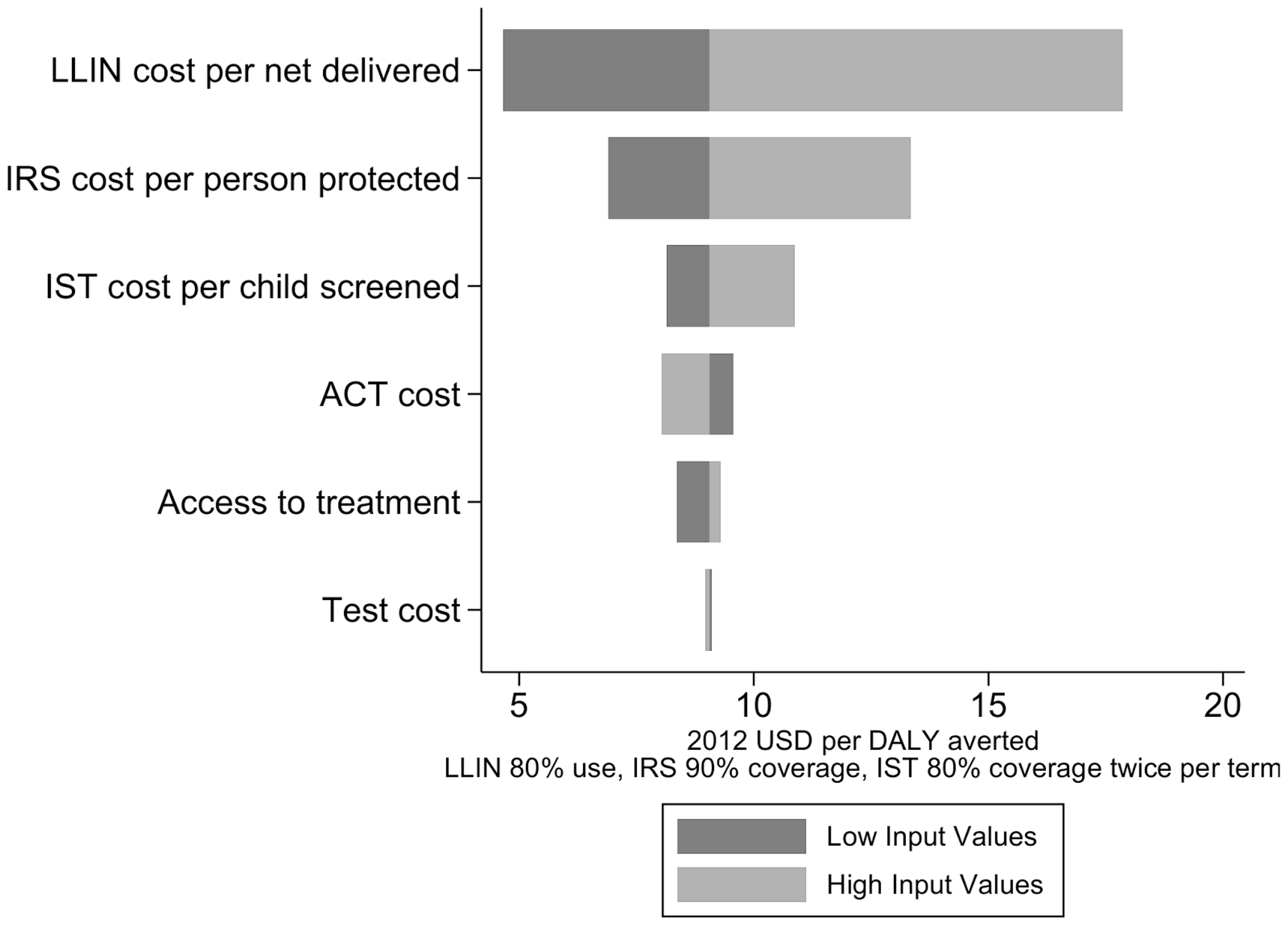
The 2011 systematic review found that, from a provider perspective, the median incremental cost effectiveness ratio per disability adjusted life year averted for ITNs was $27 for ITNs148 The "Lives Saved Tool" estimated that the cost per DALY averted thanks to malaria intervention scale-up from 2001 to 2010 in Sub-Saharan Africa was $111.
Bednets and lymphatic filariasis
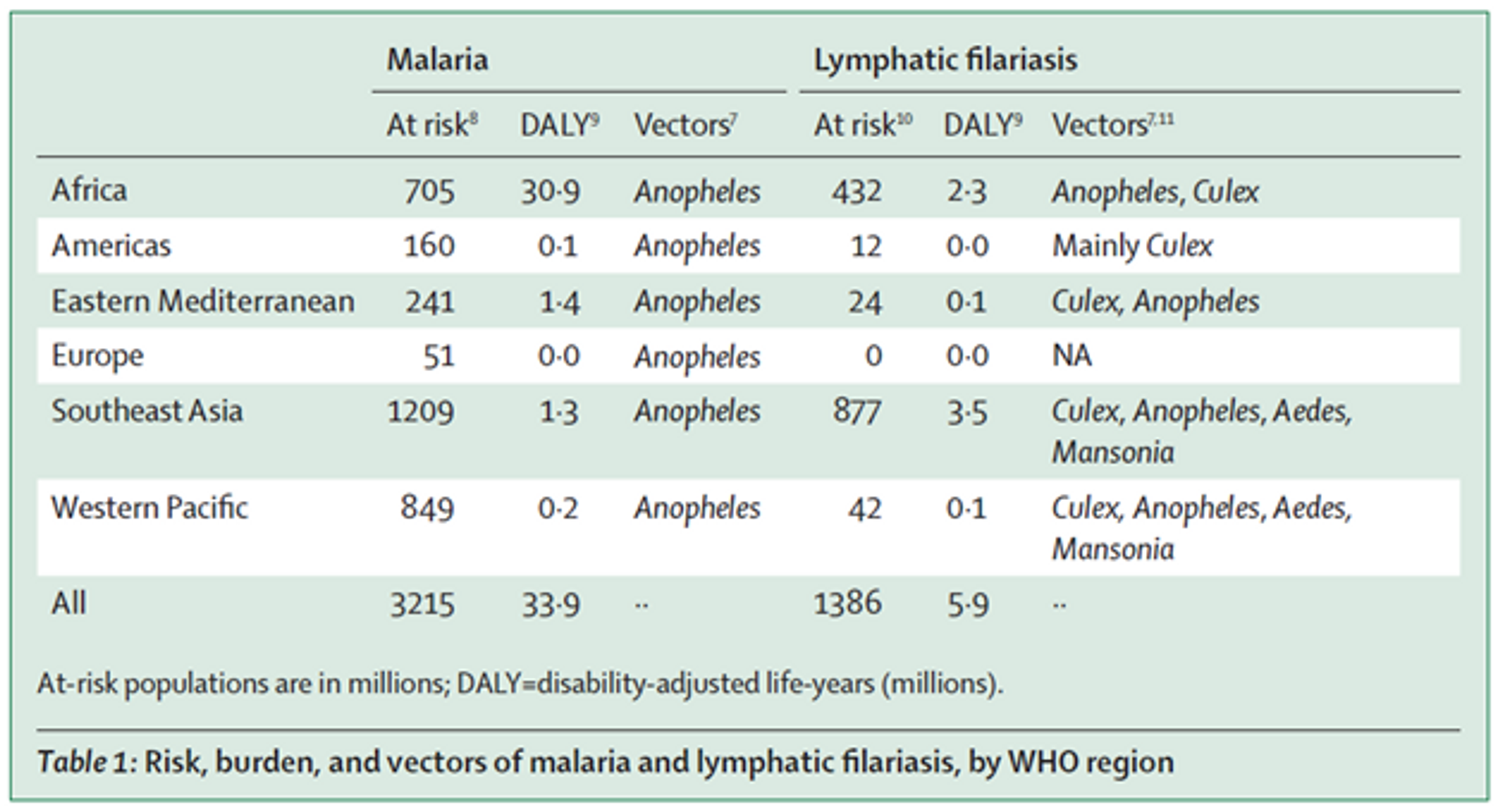
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) caused by parasitic worms. Severe disfigurement is a common symptom.149 LF is transmitted, amongst other vectors, by the same type of mosquito as malaria (see Table 1 (adapted from150). There is some evidence that suggests that bednet distributions are effective at reducing prevalence of lymphatic filariasis151152 and synergistic effects of using nets to prevent both malaria and lymphatic filariasis have been investigated.153154 For this reason, Nigeria has recently launched the first nationwide lymphatic filariasis and malaria co-implementation plan, which incorporates distribution of long-lasting insecticidal nets155 and bednet distributions have been suggested to halt transmission of LF in Nigeria.156 In Gambia, LF elimination was achieved only through bednet distributions even without any other intervention such as mass drug administration.157 A similar effect was seen in Kenya, where LF infection prevalence went down after bednet distributions even though mass drug administration rounds were missed 158 and LF elimination can be achieved with even after mass drug administration is discontinued.159160 However, the exact impact of bednets on LF is hard to quantify.161 Some modelling data suggest that if bednet coverage is sustained for a long time it can lead to local elimination of LF, even at lower levels of coverage162 and other modelling data suggests that even moderate levels of coverages can lead to a dramatic decrease of LF in the time to reach elimination. One recent paper estimated the cost effectiveness of mass drug administration for LF elimination and eradication, which might be comparable to bednet distributions for LF elimination, and found it to be very cost-effective at $72.94 - $219 per DALY averted, depending on scale up.163 Note that these cost effectiveness estimates are often very rough and should not be taken literally, however, they might indicate that LF elimination is likely to be quite cost effective.
Figure 7: Risk, burden, and vectors of malaria and lymphatic filariasis, by WHO region164
Bednets and leishmaniasis
A recent paper also suggests that it is likely that the distribution of ITNs will have positive effects on the prevention of cutaneous leishmaniasis, in areas in which the disease is co-endemic with malaria. However, evidence on the precise effect of ITN on cutaneous leishmaniasis is limited, and more research needs to be conducted in the area.165
Possible offsetting/negative impacts
We are confident that LLIN distribution does not have a substantial disruptive effect on the wider economic and health system. We address some common concerns here.
Insecticide resistance
Resistance is a serious and growing problem in malaria treatment. Out of 78 countries that reported data since 2010, 60 reported resistance to at least one insecticide in one malaria vector from one collection site, and 49 reported resistance to insecticides from two or more insecticide classes.166
Figure 8: Reported pyrethroid resistance status of malaria vectors, measured with insecticide bioassays since 2010167
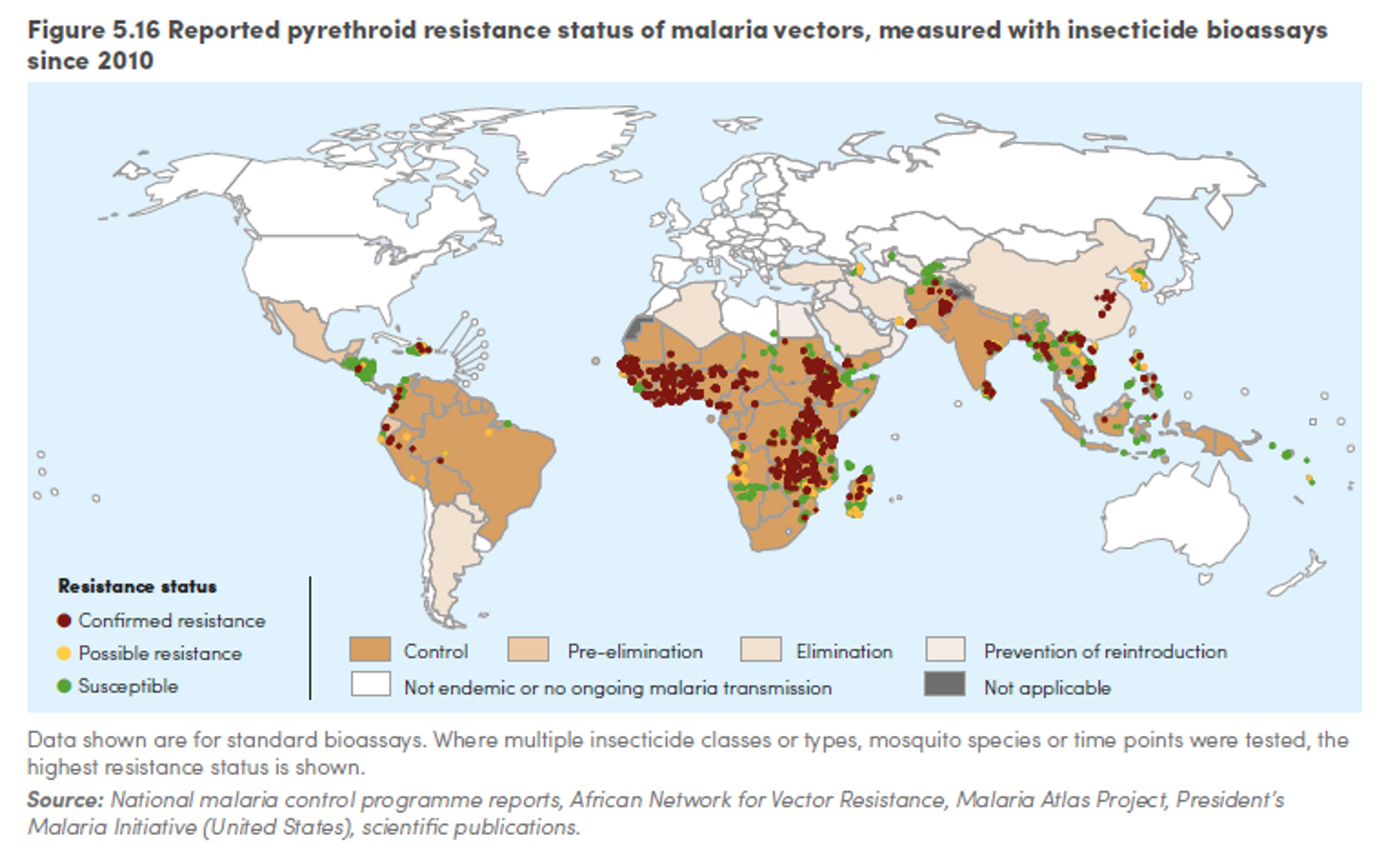
However, a meta-analysis concluded that LLINs are still more effective than nets that are not treated with insecticides regardless of resistance168 and distributing nets remains a cost-effective health intervention, even in areas with strong insecticide resistance.169 The WHO recommends that, even in areas where resistance has been identified, countries should continue to scale up or maintain universal coverage with LLINs, since the nets work as a physical barrier against mosquitoes and, even if not lethal, the insecticide is likely to contribute to malaria control.170 We refer the interested reader to a recent Givewell conversation note with an expert on insecticide resistance for more detailed information on this topic.171
The effectiveness of bed nets is also threatened by changes in biting behaviour- mosquitoes start biting during the day, when people are not protected by nets.172 Several studies have observed this change.173174175 For instance, a study in rural southern Tanzania compared mosquito biting behaviour in 1997, before bed nets were introduced, and 2009, where ITN coverage was 47%. It found a shift from a tendency to bite humans inside houses late at night toward a greater proportion occurring outdoors and around dusk or dawn.176 However, the authors note that bed net use has reduced malaria transmission by 94% in the area studied, and that it is only the residual transmission which will not be affected by further indoor insecticidal measures. The authors conclude that the findings should not undermine confidence in ITN use. A study from Benin shows evidence mosquitoes change biting behavior after the implementation of LLIN at universal coverage.177 Relatedly, the use of bednets might lead to change in species composition. A study from Senegal found that LLINs distribution favoured species that are able to feed outdoors and on cattle and that, after two years, a species whose proportion had previously decreased, was resurging, possibly having developed insecticide resistance. Once again, however, the authors stressed that the results did not mean LLIN had failed, since they had reduced the densities of the vectors that are most often in contact with human hosts.178
Finally, Part of the nets funded by AMF will be LLINs treated with a pyrethroid insecticide and the synergist piperonyl butoxide (PBO). Some evidence suggests this type of net might be more effective against mosquitoes developing resistance to pyrethroid (the insecticide generally employed on LLINs).179180. The AMF distribution in Uganda will be the first large-scale distribution using the new type of LLINs.181
Free bednets vs. selling bednets
It has been hypothesized that people who purchase nets will use them more than those who receive them for free and thus that free bednet distributions are not as good as selling them. A recent Cochrane meta analysis182 reviewed the evidence on this and found that there is probably little or no difference in net use among those who receive a free net compared to those who pay for one. They suggest that providing free insecticide-treated bednets probably increases the number of people who own bednets compared to providing subsidized bednets or bednets offered at full market price.183 Another study showed that children who receive their nets through NGOs or government likely receive some education about bednets and are more likely to use them in comparison to those children who receive nets that are bought privately through private health centers, market, shops and street vendors.184
Two recent studies tried to answer the question of whether providing free bednets affects private net sales negatively. However, evidence on the matter is conflicting. One study showed 34% monthly decline immediately after a free bednet distribution campaign compared to ‘normal’ sales.185 They found that after 6 months, the total unsold nets reached 45% of normal sales, or 347,000 nets nationwide in Tanzania. They caution that free campaigns can hinder private net sales much more than previously observed in recent experimental trials and thus can cause trouble with continuous coverage delivery channels of bednets through private sales. In contrast, another study also in Tanzania, showed distribution of free insecticide-treated nets do not interfere with continuous net distribution.186 The authors argue that the discrepancy between the two studies is likely because they used to different data from different time points. A 2014 paper found that a one-time, targeted subsidy on the long-run adoption of a new antimalarial bed net increased short-run adoption rates among both subsidy recipients and their neighbors, and subsequently increase willingness to pay for bednets.187
Even if stronger evidence supported the thesis that free distributions of nets displace private sales, there are still strong reasons in favour of distributing bednets for free (as recommended by the WHO) rather than charging for them. First, bednets do not only benefit the user but also others in their community by reducing the rate of infection. Second, evidence shows that charging even small amounts for bednets decreases coverage rate by about 75%.188
Bednets and reduced malaria immunity
In the 1990, there had been some debate in the academic literature that by protecting children from malaria (through bednets), one will only reduce the short-term burden of malaria, but people will not acquire immunity to malaria in childhood and thus malaria will increase in the long-term.189 This “delayed mortality” hypothesis has since been discredited and there is a strong consensus among malaria experts that there is no delayed mortality effect due to lack of evidence.190
Misuse of bednets
In the last years, a few reports have suggested that bed nets had been used for fishing 191192193 and a 2015 article in the New York Times194 “claimed countless [people in Africa] are not using their mosquito nets as global health experts have intended,” most often for fishing, which causes harm to fish stock due to the nets insecticides and fine gauges.
We could only find one study that quantified the extent of bednet use in fishing: a small survey study with 196 respondents in seven villages surrounding a Lake Tanganyika reported that 87% of households surveyed have used a mosquito bed net for fishing at some point. However, another much more comprehensive analysis of 14 surveys in several countries with 14,196 households showed that that the overwhelming majority of nets were used for malaria prevention, and only 255 nets were repurposed (which make up less than 1% overall). Furthermore, the majority of the repurposed nets were already considered too torn, indicating they had already served out their useful life for malaria prevention.195 The authors conclude that national programmes and donor agencies should remain confident in the appropriate use of bednets.
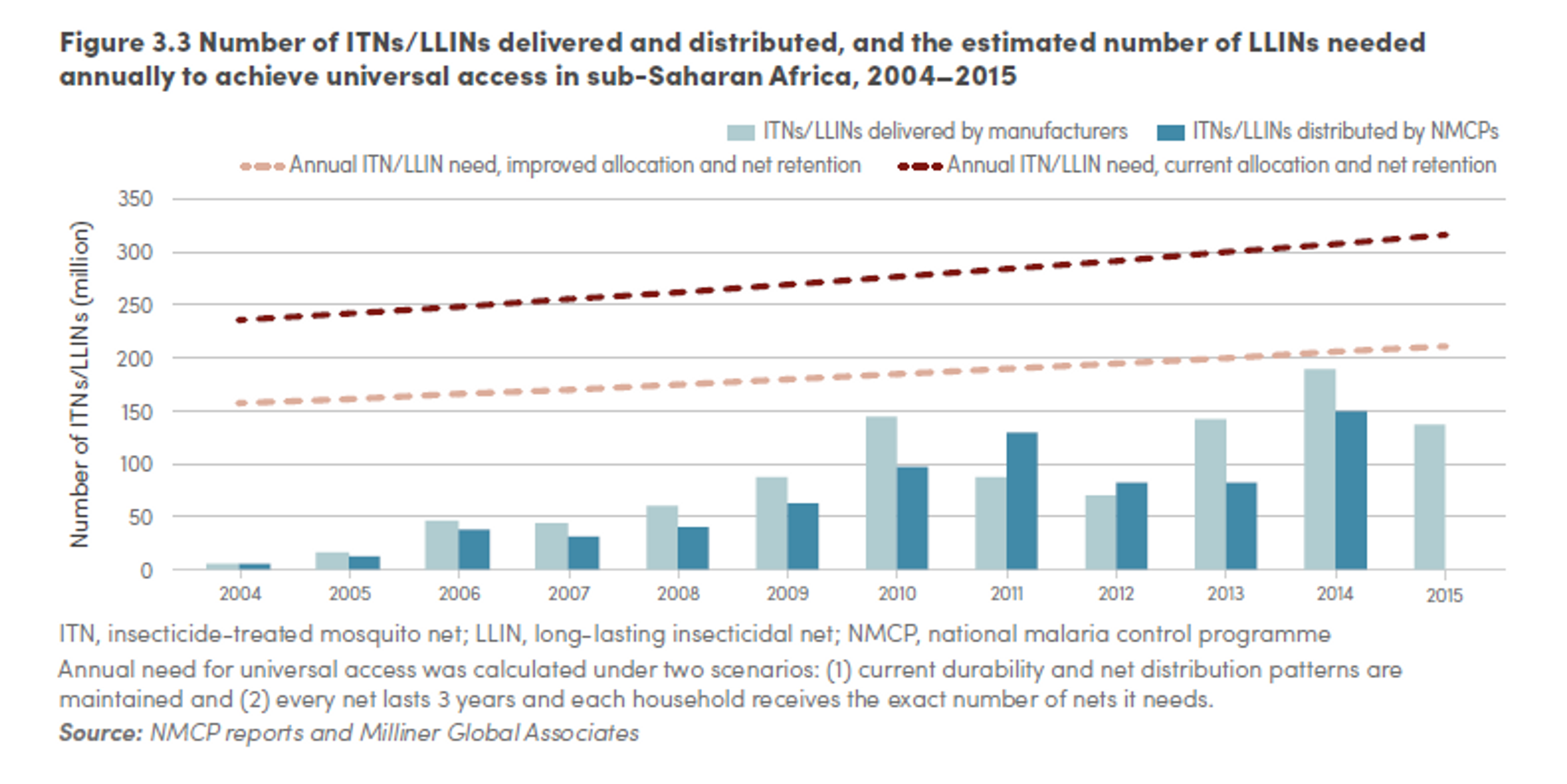
3. Neglectedness
It is not easy to estimate the global LLINs gap. The WHO’s Roll Back Malaria Harmonization Working group has recently updated their estimates of the net gap for Sub-Saharan Africa.196 They estimate that in 2015 there was a gap of about 39 million nets. They suggests that the gap in 2016 will be around 64 million nets, which translates to a funding gap of about 343 million dollars (for our calculation see our excel spreadsheet197 and Table 1).
| LLIN Commodity Gaps | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|
| Need | 227,448,317 | 179,115,337 | 245,651,438 | 222,611,818 |
| Financed | 188,242,201 | 114,443,209 | 104,753,670 | 15,577,419 |
| Gap | 39,206,116 | 64,672,128 | 140,897,768 | 207,034,399 |
| LLIN Funding Gaps | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|
| Need | $1,207,750,563.27 | $951,102,439.47 | $1,304,409,135.78 | $1,182,068,753.58 |
| Financed | $999,566,087.31 | $607,693,439.79 | $556,241,987.70 | $82,716,094.89 |
| Gap | $208,184,475.96 | $343,408,999.68 | $748,167,148.08 | $1,099,352,658.69 |
Table 1 : Overall commodity and funding gaps for bednets, based on GiveWell's estimate of the average cost per net in the countries that AMF is considering future distributions - $5.31 (also using 198)
However, the WHO estimates indicate a much larger gap. The WHO estimates that, if nets were allocated to households with maximum efficiency and nets were retained in households for at least three years, 200 million nets are required each year to achieve universal access. However, the number is higher if we take into account the current distribution patterns of nets in households and the loss of nets estimated from distribution and survey data. Under these more realistic assumptions, as many as 300 million new nets would be required each year to ensure that all persons at risk of malaria had access to an LLIN in countries in which the use of LLINs is the primary method of vector control. Employing the Roll Back Malaria estimates of the number of financed bed nets, this translates in a gap of about 112 million nets in 2015.
In sum, it is difficult to estimate the global funding gap for LLINs, but evidence suggests that, at the minimum, in 2016 there is likely to be a gap of 64 million nets (343 million dollars).
Figure 9: Number of ITNs/LLINs delivered and distributed, and the estimated number of LLINs needed annually to achieve universal access in sub-Saharan Africa, 2014-2015199
The above estimates are calculated on the assumption that net durability is about three years. However, this assumption has recently been challenged. Insecticide treated nets with holes in them are less effective at protecting people from malaria than intact nets.200 In line with this, monitoring of durability of nets in Rwanda showed greater than anticipated bednet loss, associated with poor fabric integrity, during year two of a three year LLIN distribution-replacement cycle. The proportion of the remaining nets in need of replacement, after two years, was large enough to suggest that the intervention would lose impact during year three of the distribution-replacement cycle201 and this might be one of the reasons for resurgence of malaria in Rwanda in 2009, following a 2006 under-five, country-wide bed net campaign. Similarly, another study suggests that bednet distribution conducted every three years, which is a key intervention of Benin’s malaria control strategy, is insufficient and a two-year serviceable life for the current LLIN intervention would be a more realistic program assumption.202
Importantly, durability seems to change across sites. A cross-sectional study from Northeast India found that that the serviceable life of the nets was slightly less than three years in terms of waning residual bio-efficacy and durability that warranted replacement.203 A recent study from Tanzania found that, 2–4 years after a mass campaign, only 39% of distributed nets remain both present and in serviceable physical condition, which is below WHO assumptions of 50% survival after three years.204 A recent study from Zambia found that the median functional survival time for LLINs observed the study was 2.5–3 years and insecticide activity and content were markedly decreased by two years.205 A study undertaken in three states in Nigeria found that the proportion of surviving nets in serviceable condition differed dramatically, resulting in an estimated median net survival of 3 years in Nasarawa, 4.5 years in Cross River and 4.7 years in Zamfara.206
It should be stressed that, if net durability is lower than previously thought, more nets might be needed than previously assumed.[^fn-1]: "WHO | Malaria." 2004. 2 Feb. 2016 <http://www.who.int/mediacentre/factsheets/fs094/en/>